


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 1-12-2015
Date: 14-4-2021
Date: 22-4-2021
|
23S rRNA Has Peptidyl Transferase Activity
KEY CONCEPT
- Peptidyl transferase activity resides exclusively in the
23S rRNA.
The sites involved in the functions of 23S rRNA are less well identified than those of 16S rRNA, but the same general pattern is observed: Bases at certain positions affect specific functions. Bases at some positions in 23S rRNA are affected by the conformation of the A site or the P site. In particular, oligonucleotides derived from the 3′ CCA terminus of tRNA protect a set of bases in 23S rRNA that essentially are the same as those protected by peptidyl-tRNA. This suggests that the major interaction of 23S rRNA with peptidyl-tRNA in the P site involves the 3′ end of the tRNA.
The tRNA makes contact with the 23S rRNA in both the P and A sites. At the P site, G2552 of 23S rRNA base pairs with C74 of the peptidyl tRNA. A mutation in the G in the rRNA prevents interaction with tRNA, but interaction is restored by a compensating mutation in the C of the amino acceptor end of the tRNA. At the A site, G2553 of the 23S rRNA base pairs with C75 of the aminoacyltRNA.
Thus, rRNA plays a close role in both the tRNA-binding sites. As structural studies continue to emerge, the movements of tRNA between the A and P sites in terms of making and breaking contacts with rRNA will be elucidated.
Another site that binds tRNA is the E site, which is localized almost exclusively on the 50S subunit. Bases affected by its conformation can be identified in 23S rRNA. What is the nature of the site on the 50S subunit that provides peptidyl transferase function? A long search for ribosomal proteins that might possess the catalytic activity was unsuccessful and led to the discovery that the ribosomal RNA of the large subunit can catalyze the formation of a peptide bond between peptidyl-tRNA
and aminoacyl-tRNA. The involvement of rRNA was first indicated because a region of the 23S rRNA is the site of mutations that confer resistance to antibiotics that inhibit peptidyl transferase. Extraction of almost all the protein content of 50S subunits leaves the 23S rRNA largely associated with fragments of proteins, amounting to less than 5% of the mass of the ribosomal proteins.
This preparation retains peptidyl transferase activity. Treatments that damage the RNA abolish the catalytic activity. Following from these results, 23S rRNA prepared by transcription in vitro can catalyze the formation of a peptide bond between Ac-Phe-tRNA and Phe-tRNA. The yield of Ac-Phe-Phe is very low, suggesting that the 23S rRNA requires proteins in order to function
at a high efficiency. However, given that the rRNA has the basic catalytic activity, the role of the proteins must be indirect, serving to fold the rRNA properly or to present the substrates to it. The reaction also works, although less effectively, if the domains of 23S rRNA are synthesized separately and then combined. In fact, some activity is shown by domain V alone, which has the catalytic center. Activity is abolished by mutations in position 2252 of domain V that lies in the P site.
The crystal structure of an archaeal 50S subunit shows that the peptidyl transferase site basically consists of 23S rRNA. No protein exists within 18 Å of the active site where the transfer reaction occurs between peptidyl-tRNA and aminoacyl-tRNA! Peptide bond synthesis requires an attack by the amino group of one amino acid on the carboxyl group of another amino acid.
Catalysis requires a basic residue to accept the hydrogen atom that is released from the amino group, as shown in Figure 1. If rRNA is the catalyst, it must provide this residue, but it is not known how this happens. The purine and pyrimidine bases are not basic at physiological pH. A highly conserved base (at position 2451 in E. coli) had been implicated but appears now neither to have the right properties nor to be crucial for peptidyl transferase activity.
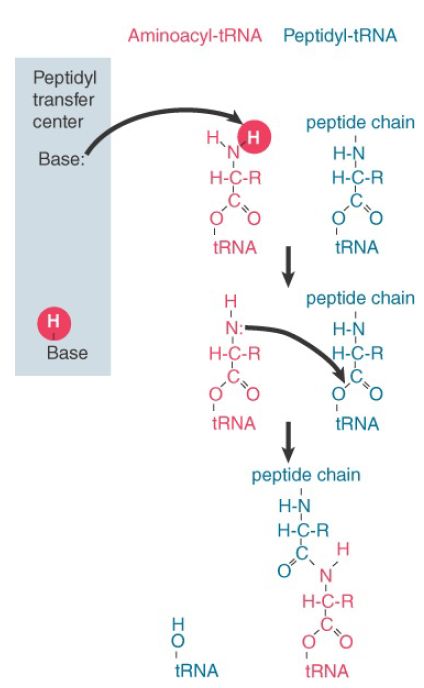
FIGURE 1 . Peptide bond formation requires acid–base catalysis in which an H atom is transferred to a basic residue.
The catalytic activity of isolated rRNA is quite low, and proteins that are bound to the 23S rRNA outside of the peptidyl transfer region are almost certainly required to enable the rRNA to form the proper structure in vivo. The idea that rRNA is the catalytic component is consistent with the results discussed in the RNA Splicing and Processing chapter, which identify catalytic properties in RNA that are involved with several RNA-processing reactions. It fits with the notion that the modern ribosome evolved from a prototype originally composed solely of RNA.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|