


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Checkpoint Control for Entry into S Phase: p53, a Guardian of the Checkpoint |
|
|
|
Read More
Date: 7-4-2021
Date: 29-12-2015
Date: 17-5-2016
|
Checkpoint Control for Entry into S Phase: p53, a Guardian of the Checkpoint
KEY CONCEPTS
- The tumor suppressor proteins p53 and Rb act as guardians of cell integrity.
- A set of ser/thr protein kinases called cyclin-dependent kinases control cell cycle progression.
- Cyclin proteins are required to activate cyclin-dependent kinase proteins.
- Inhibitor proteins negatively regulate the cyclin/cyclindependent kinases.
- Activator proteins called CDK-activating kinases positively regulate the cyclin/cyclin-dependent kinases.
Progression through the cell cycle, after the initial activation by growth factor, requires continuous growth factor presence and is tightly controlled by a second set of ser/thr protein kinases called cyclin-dependent kinases (CDKs; and sometimes cell division–dependent kinases). The CDKs themselves are controlled in a very complex fashion as shown in FIGURE 1. They are inactive by themselves and are activated by the binding of cell cycle–specific proteins called cyclins. This means that the CDKs can be synthesized in advance and left in the cytoplasm. In addition to cyclins, the CDKs are regulated by multiple phosphorylation events.
One set of kinases, the Wee1 family of ser/thr kinases, inhibits the CDKs, while another, the CDK-activating kinases (CAKs), activates them. (Wee1 kinases inhibit cell cycle progression, and if they are mutated, premature cell cycle progression results in wee, tiny cells.) This also means that the balance of kinases and phosphatases regulates the activity of the CDKs. We will focus on the G1 to S phase transition. (There is similar tight control at the G2 to M transition and within various stages of mitosis and meiosis.) The signal for entry into S phase is a positive signal controlled by negative regulators. The S to G2 transition occurs when replication is completed.

FIGURE 1. Formation of an active CDK requires binding to a cyclin. The process is regulated by positive and negative factors.
For a cell to be allowed to progress from G1 to S phase, two major requirements must be met. The cell must have grown a specific amount in size and there must be no DNA damage. The worst thing that a cell can do is to replicate damaged DNA. To ensure that both requirements are met, the CDK/cyclin complexes are controlled by checkpoint proteins. Two of the most important are the transcription factors p53 and Rb. These two proteins are in a class called tumor suppressor proteins. As guardians of the cell cycle, these proteins ensure that the cell size and absence of DNA damage criteria are met. Even in the presence of an oncogenic mutant RAS protein, tumor suppressors will prevent the cell from progressing from G1 to S; they are the brakes on the cell cycle.
Mutations in tumor suppressor proteins allow damaged and undersized cells to replicate. These recessive, loss-of-function mutations, especially in p53 and Rb, are the most common tumor suppressor mutations in tumors; frequently both are seen together. The DNA damage checkpoint controlled by p53 is the one that is best understood (FIGURE 2). The function of p53 is to relay information to the CDK/cyclins that damage has occurred to prevent entry into S phase; that is, it ultimately causes cell cycle arrest. In addition, in the event that damage is very extensive or otherwise unrepairable, p53 will initiate an alternate pathway, apoptosis, or programmed cell death (PCD). p53 transcription is upregulated by growth factor stimulation, as the cell begins preparation for its trip through G1 and the important G1 to S transition.
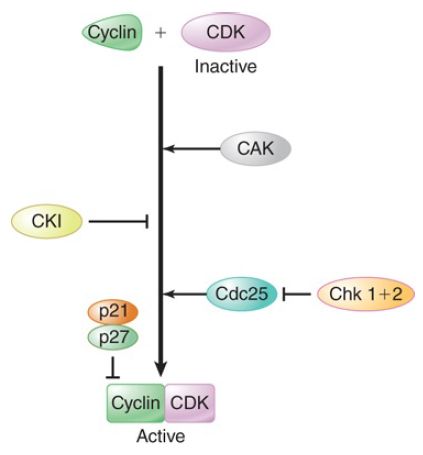
FIGURE 2. DNA damage pathway. p53 is activated by DNA damage. Activated p53 halts the cell cycle through Rb and stimulates DNA repair. p53 is regulated by a complex set of activators and inhibitors.
The p53 protein product is regulated by multiple complex pathways. The major regulator is a protein called MDM2, which works through a negative feedback loop. MDM2 transcription is increased by p53, and it in turn inhibits p53 in a positive feedback loop, by targeting it to the ubiquitin-dependent proteosomal degradation pathway, as described further in the section Checkpoint Control for Entry into S Phase: Rb, a Guardian of the Checkpoint coming up next. It also binds to p53 and prevents it from activating transcription. DNA damage leads to phosphorylation of MDM2, which inhibits its ability to promote p53 degradation, allowing p53 levels to increase. Growth factor stimulation of cell cycle progression also leads to an increase in transcription of the p19ARF protein (p14 in humans), which binds to and inhibits MDM2’s ability to inhibit p53. The human p14ARF is transcribed from an interesting genetic locus, the INK4a/ARF locus, which gives rise to three proteins by alternative splicing and alternative promoter usage: p15INK , p16INK , and p14ARF (ARF stands for alternate reading frame).
p53 is activated by DNA damage or different kinds of stress through a protein kinase relay system from the nucleus that ultimately phosphorylates and stabilizes p53 from degradation. This leads to an increased level of p53 and activates its ability to serve as a transcription factor to turn on some genes and repress other genes. Among those genes turned on are GADD45 to stimulate DNA repair; p21/WAF-1, whose product binds to and inhibits the CDK/cyclin complexes for G1 arrest (or promotes apoptosis if the DNA damage is too great); sets of large intergenic noncoding RNAs (lincRNAs) to mediate transcription repression; and miRNAs (as described in the chapter titled Regulatory RNA). A specific lincRNA, p21-lincRNA, mediates the repressive properties of p53 by binding to specific chromatin complexes.
DNA damage also independently activates a pair of protein kinases, Chk1 and Chk2, which phosphorylate and inhibit CDKs, and phosphorylate and inhibit the phosphatase Cdc25 (cell division cycle), which is required to activate the CDKs.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|