
SPIN PRECESSION AND SPIN RESONANCE
 المؤلف:
Thomas Banks
المؤلف:
Thomas Banks
 المصدر:
Quantum Mechanics
المصدر:
Quantum Mechanics
 الجزء والصفحة:
190
الجزء والصفحة:
190
 27-3-2021
27-3-2021
 3436
3436
SPIN PRECESSION AND SPIN RESONANCE
Electron spin resonance (ESR) is a technique that uses the simple Zeeman effect to identify materials with unpaired electrons. It has applications throughout chemistry and biology. It is closely related to nuclear magnetic resonance (NMR), where the splitting of nuclear spin levels in an external field is used. Nuclear magnetic moments are inversely proportional to nucleon masses, and are thus � 2, 000 times smaller than the electron moment. For a fixed magnetic field, the energy splittings are thus much smaller in the nuclear case, which means that NMR signals involve electromagnetic radiation of much lower frequency than ESR signals.
The basic idea of ESR or NMR experiments is to subject a sample containing a large number of atoms with the same unpaired electron/nuclear spin to both a source of monochromatic radiation and an external magnetic field B0. The magnetic field produces a splitting ΔE = gμBB0 between two spin states (here g is a g-factor, which might be different than the free electron ge ∼ 2 because of the environment in which the unpaired electron sits). At finite temperature, the average number of electrons in each state will differ, by a Boltzmann factor e−ΔE/kT. One then tunes the field B0 so that ΔE = hω, the photon energy of the monochromatic beam. At this resonance frequency the electrons can move between the higher and lower state by (stimulated) emission or absorption of a photon. Since the lower state is more heavily populated, this will give rise to a net absorption of the radiation, which we can monitor by detecting the beam after it is passed through the sample. For microwaves of frequency ∼ 9, 390 MHz, the resonance (for g = ge) occurs at B0 ∼ .335Tesla, while for the same magnetic field, NMR occurs for a frequency of about 14 MHz.
ESR signals can give information about the atomic and molecular structure of the compounds in which the electrons that produce the response are embedded. For example, as we noted above, LS coupling can change the nature of the electronic spin states and correlate them with the orbital state of the electron. This effect leads to the replacement of the free electron ge factor by a matrix. The i-th component of the magnetic moment is given by
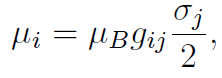
and ESR experiments measure gij . There are also interesting complications introduced by interaction of the electron spin with nuclear spins, and these can also be exploited to learn about the structure and composition of the materials in which the electron is embedded.
 الاكثر قراءة في ميكانيكا الكم
الاكثر قراءة في ميكانيكا الكم
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


