

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-7-2020
Date: 4-11-2020
Date: 12-8-2020
|
When methane gas is combusted, heat is released, making the reaction exothermic. Specifically, the combustion of 1mol of methane releases 890.4 kilojoules of heat energy. This information can be shown as part of the balanced equation.
The equation tells us that 1mol of methane combines with 2mol of oxygen to produce 1mol of carbon dioxide and 2mol of water. In the process, 890.4kJ is released and so it is written as a product of the reaction. A thermochemical equation is a chemical equation that includes the enthalpy change of the reaction. The process in the above thermochemical equation can be shown visually in Figure 1
.
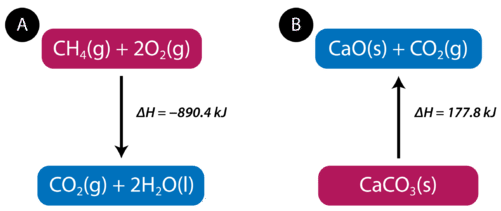
Figure 1 : (A) As reactants are converted to products in an exothermic reaction, enthalpy is released into the surroundings. The enthalpy change of the reaction is negative. (B) As reactants are converted to products in an endothermic reaction, enthalpy is absorbed from the surroundings. The enthalpy change of the reaction is positive.
In the combustion of methane example, the enthalpy change is negative because heat is being released by the system. Therefore, the overall enthalpy of the system decreases. The heat of reaction is the enthalpy change for a chemical reaction. In the case above, the heat of reaction is −890.4kJ
. The thermochemical reaction can also be written in this way:
Heats of reaction are typically measured in kilojoules. It is important to include the physical states of the reactants and products in a thermochemical equation as the value of the ΔH
depends on those states. Endothermic reactions absorb energy from the surroundings as the reaction occurs. When 1mol of calcium carbonate decomposes into 1mol of calcium oxide and 1mol of carbon dioxide, 177.8kJ of heat is absorbed. The process is shown visually in Figure 1B . The thermochemical reaction is shown below.
Because the heat is absorbed by the system, the 177.8kJ is written as a reactant. The heat of reaction is positive for an endothermic reaction.
The way in which a reaction is written influences the value of the enthalpy change for the reaction. Many reactions are reversible, meaning that the product(s) of the reaction are capable of combining and reforming the reactant(s). If a reaction is written in the reverse direction, the sign of the ΔH
changes. For example, we can write an equation for the reaction of calcium oxide with carbon dioxide to form calcium carbonate.
The reaction is exothermic and thus the sign of the enthalpy change is negative.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة لجامعة سومر للمشاركة في حفل التخرج المركزي الخامس
|
|
|