
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-5-2016
Date: 23-10-2019
Date: 26-1-2016
|
Reaction of thionyl chloride with chiral 2º-alcohols: Mechanisms
Since the reaction proceeds through a backside attack (SN2 ), there is inversion of configuration at the carbon
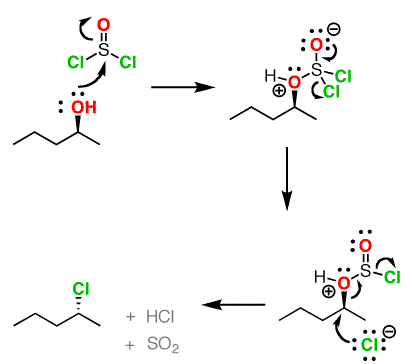
The mechanism for formation of acid chlorides from carboxylic acids is similar. The conversion of caboxylic acids to acid chlorides is similar, but proceeds through a [1,2]-addition of chloride ion to the carbonyl carbon followed by [1,2]-elimination to give the acid chloride, SO2 and HCl.
The PBr3 reaction is thought to involve two successive SN2-like steps:
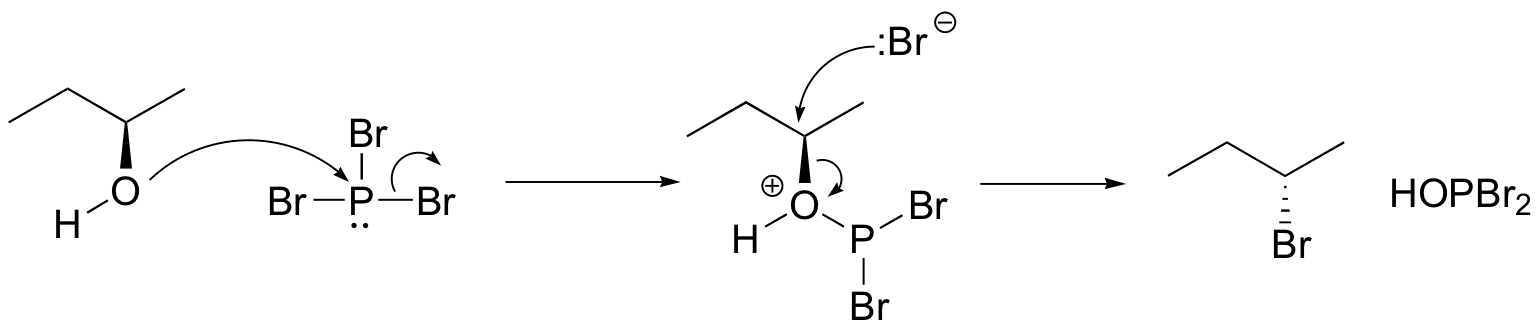
Notice that these reactions result in inversion of stereochemistry in the resulting alkyl halide.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|