


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-7-2019
Date: 11-1-2020
Date: 3-7-2019
|
The most commonly used procedure for separating enantiomers is to convert them to a mixture of diastereomers that will have different physical properties: melting point, boiling point, solubility, and so on . For example, if you have a racemic or D,L mixture of enantiomers of an acid and convert this to a salt with a chiral base having the D configuration, the salt will be a mixture of two diastereomers, (D acid . D base) and (L acid . D base). These diastereomeric salts are not identical and they are not mirror images. Therefore they will differ to some degree in their physical properties, and a separation by physical methods, such as crystallization, may be possible. If the diastereomeric salts can be completely separated, the acid regenerated from each salt will be either exclusively the D or the L enantiomer:
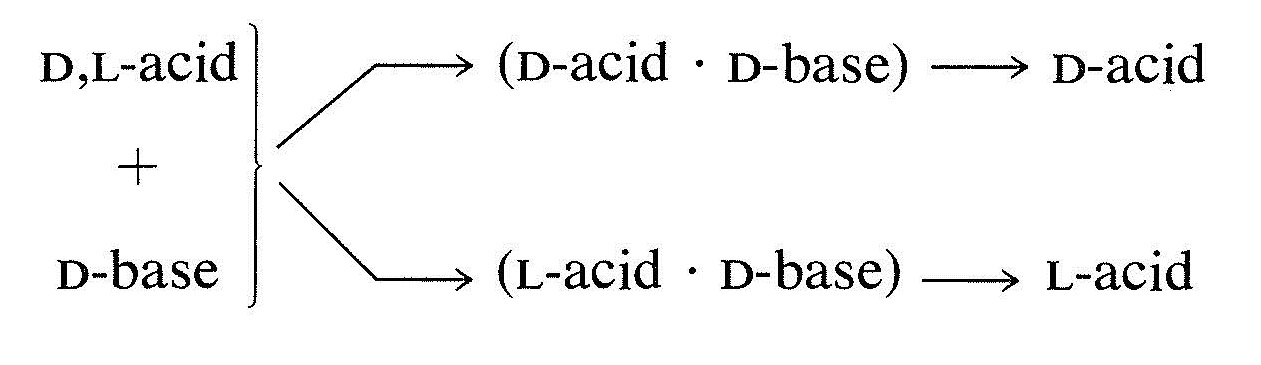
Resolution of chiral acids through the formation of diastereomeric salts requires adequate supplies of suitable chiral bases. Brucine, strychnine, and quinine frequently are used for this purpose because they are readily available, naturally occurring chiral bases. Simpler amines of synthetic origin, such as 2-amino- 1 -butanol, amphetamine, and 1 -phenylethanamine, also can be used, but first they must be resolved themselves.
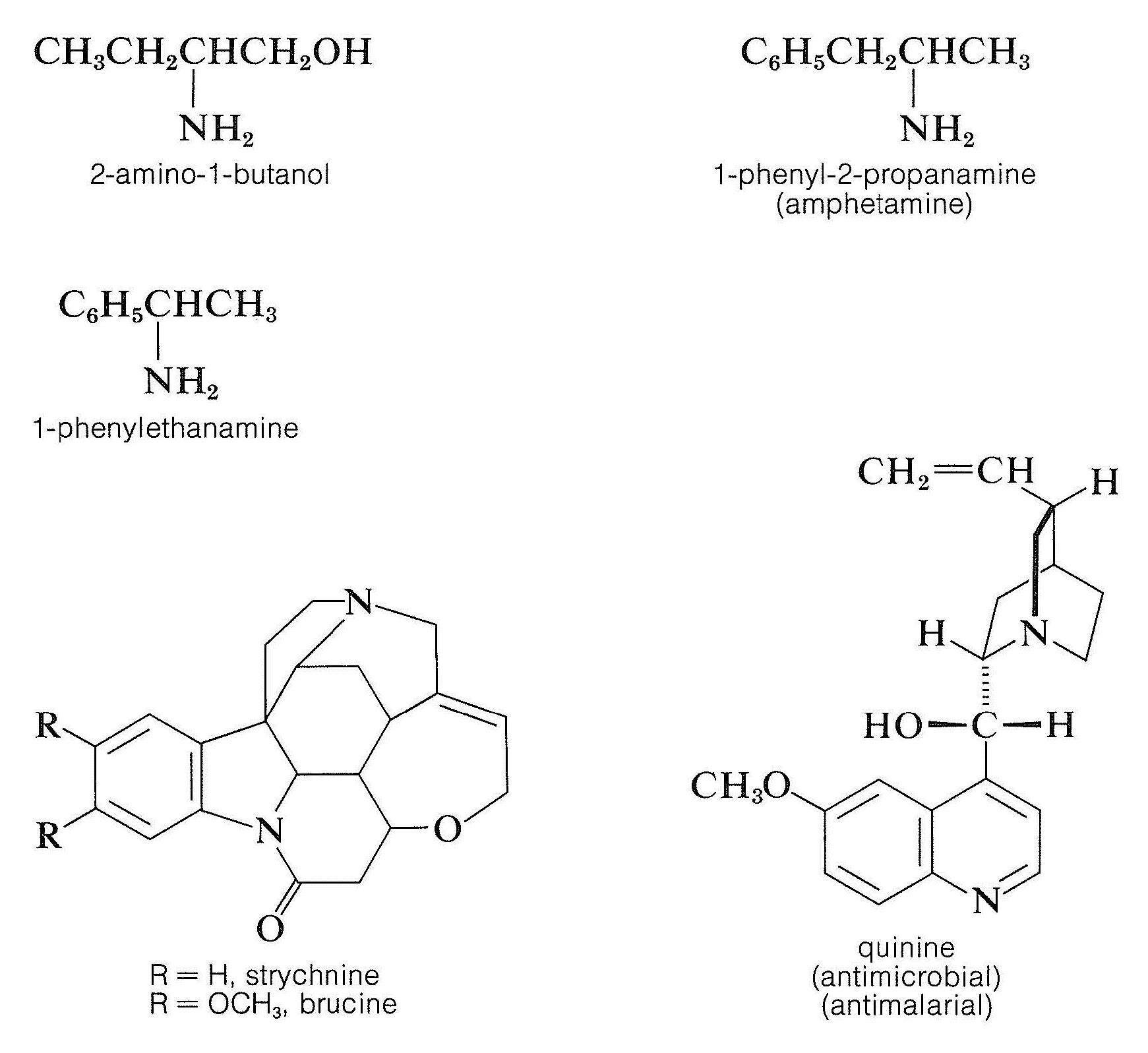



|
|
|
|
علامات تحذيرية قد تسبق الموت القلبي المفاجئ لدى الشباب
|
|
|
|
|
|
|
استنساخ ذئاب عملاقة وشرسة "انقرضت منذ آلاف السنين"
|
|
|
|
|
|
|
أصواتٌ قرآنية واعدة .. أكثر من 80 برعماً يشارك في المحفل القرآني الرمضاني بالصحن الحيدري الشريف
|
|
|