


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-1-2019
Date: 12-1-2018
Date: 29-11-2018
|
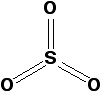
Pure sulfur trioxide is a white solid with a low melting and boiling point. It reacts very rapidly with water vapour in the air to form sulfuric acid. That means that if you make some in the lab, you tend to see it as a white sludge which fumes dramatically in moist air (forming a fog of sulfuric acid droplets). Gaseous sulfur trioxide consists of simple SO3 molecules in which all six of the sulfur's outer electrons are involved in the bonding.
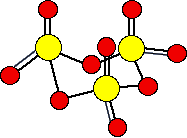
There are various forms of solid sulfur trioxide. The simplest one is a trimer, S3O9, where three SO3 molecules are joined up and arranged in a ring.
There are also other polymeric forms in which the SO3 molecules join together in long chains. For example:
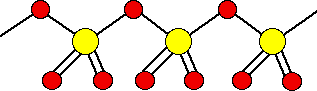
It is difficult to draw this convincingly. In fact, on each sulfur atom, one of the double bonded oxygens is coming out of the diagram towards you, and the other one is going back in away from you.
The fact that the simple molecules join up in this way to make bigger structures is what makes the sulfur trioxide a solid rather than a gas.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|