
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-9-2018
Date: 30-9-2018
Date: 30-9-2018
|
Using half-life period for determine the order of a reaction
Two separate experiments are performed by taking different initial concentrations of a reactant.
The progress of the reaction in each case is recorded by analysis. When the initial concentration is reduced to one-half, the time is noted. Let the initial concentrations in the two experiments be [A1] and [A2], while times for completion of half change are t1and t2 respectively.
Calculation of order of reaction. We know that half-life period for a first order reaction is independent of the initial concentration, [A]. We also know :
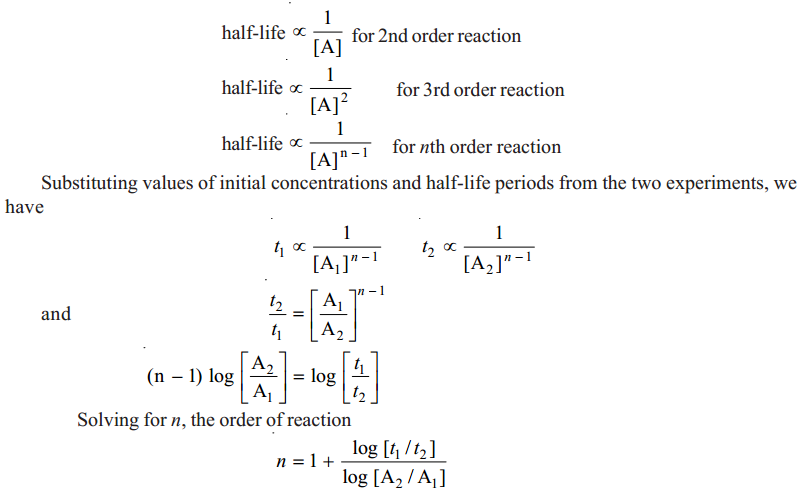



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|