


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-12-2020
Date: 23-9-2018
Date: 22-9-2018
|
FIRST ORDER REACTIONS
Let us consider a first order reaction
A⎯⎯→ products
Suppose that at the beginning of the reaction (t= 0), the concentration of A is amoles litre–1
. If after time t, x moles of A has changed, the concentration of A is a– x. We know that for a first order reaction, the rate of reaction, dx/dt, is directly proportional to the concentration of the reactant. Thus,
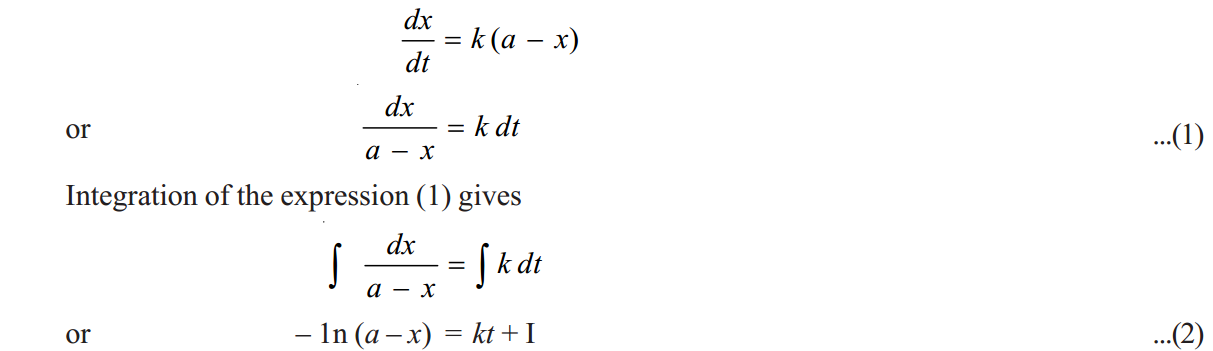
where I is the constant of integration. The constant k may be evaluated by putting t= 0 and x= 0.
Thus, I = – 1n a
Substituting for I in equation (2)
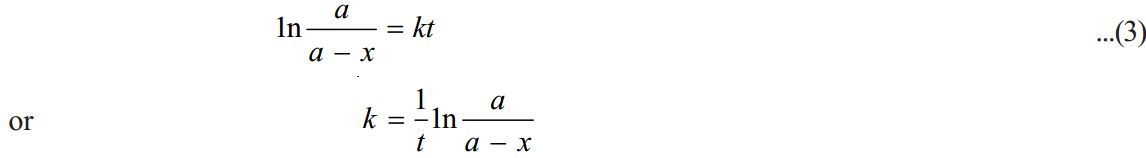
Changing into common logarithms

The value of k can be found by substituting the values of a and (a– x) determined experimentally at time interval t during the course of the reaction.
Sometimes the integrated rate law in the following form is also used :
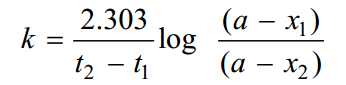
Where x1and x2 are the amounts decomposed at time intervals t1 and t2 respectively from the start.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|