


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-8-2020
Date: 4-8-2020
Date: 27-7-2020
|
Absorption of visible and/or ultraviolet light by a molecule introduces energy sufficient to break or reorganize most covalent bonds. From the relationship E = hc / λ, we see that longer wavelength visible light (400 to 800 nm) is less energetic (70 to 40 kcal/mole) than light in the accessible shorter wavelength (200 to 400 nm) near ultraviolet region (150 to 70 kcal/mole). Consequently, ultraviolet light is most often used to effect photochemical change. Care must also be taken to construct lamps and reaction vessels from glass that is transparent to the desired wavelength range. The low wavelength cut-off for some common glass types are given in the table on the right.
| Type of Glass | Wavelength Cut-off |
|---|---|
| Pyrex | < 275 nm |
| Corex | < 260 nm |
| Vycor | < 220 nm |
| Quartz | < 170 nm |
The light required for a photochemical reaction may come from many sources. Giacomo Ciamician, regarded as the "father of organic photochemistry", used sunlight for much of his research at the University of Bologna in the early 1900's. Depending on the compounds being studied and the information being sought, bright incandescent lamps (chiefly infrared and visible light), low, medium and high pressure mercury lamps (185 - 255 nm, 255 -1000 nm & 220 -1400 nm respectively), high intensity flash sources and lasers have all been used. In careful studies of specific chromophores, sources of monochromatic light may be desired.
In this section we shall focus chiefly on the nature and behavior of the electronic excited states formed when a photon is absorbed by a chromophoric functional group. As a rule, such excitation results in a change in molecular orbital occupancy, an increase in energy, and changes in local bonding and charge distribution. The principles set forth in the UV-Visible Spectroscopy chapter will provide a helpful foundation.
We begin by considering the electronic excitation of a simple diatomic molecule such as Cl2 or Br2. Both absorb light, chlorine in the 300 to 380 nm region. and bromine in the 360 to 510 nm region.
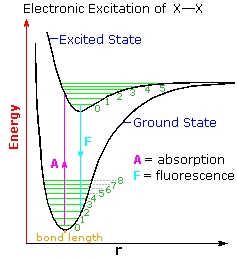
The diagram on the right illustrates the initial electronic excitation. Both the ground (lowest energy electronic state) and excited states are shown as energy profiles populated by vibrational energy states (green lines) as well as rotational states (not shown). The electron reorganization that occurs when the ground electronic state is excited by absorption of a photon takes place much more rapidly than any movement of the atom nuclei that eventually follow. In other words, electron shifts, when viewed from the perspective of the nuclear coordinates, occur as if the heavier nuclei were fixed in place. This consequence of the Born-Oppenheimer approximation led James Franck and R. Condon to formulate the Franck-Condon Principle: Electronic transitions occur much faster than nuclei can respond.
Overall bonding in an excited state is usually lower than in the ground state. Thus, the X–X bond length is increased in the excited state. At normal temperatures essentially all molecules will exist in the ground vibrational state (zero level). The Franck-Condon principle requires that excitation occur by a vertical transition, shown by the red line, resulting in the population of higher vibrational levels in the excited state. Several events may then take place.
1. The vibrational energy may be lost as heat, relaxing the excited state to its zero vibrational level.
2. The excited state may return to the ground state by emitting a photon (light blue line). If this happens from the zero vibrational level the frequency or energy of the emitted light will be lower than that of the initially absorbed light. This radiative decay is called fluorescence if it takes place rapidly from the initial excited state. It is termed phosphorescence if it occurs slowly by way of other excited states.
3. If a higher vibrational level of the excited state is populated, either by the initial Franck-Condon transition or by collisional activation, the molecule may cleave into two X• atoms. Note that vibrational level 5 of the excited state is roughly coincident with bond breaking.
Virtually all organic compounds have more than two atoms, so the potential energy state diagram of X2 must be adjusted for the increased number of bonding relationships. One way of doing this is to retain the energy coordinate while dispensing with the dimensional (r) coordinate. The Jablonski diagram shown below is an example, in which the spatial orientation of the various electronic states is not specified. Nevertheless, Franck-Condon transitions are expected.
One important feature conveyed by the diagram is that more than one electronic excited state is likely to exist for a given molecule, six are drawn and labeled in the diagram. Each electronic state will have a group of vibrational (and rotational) states, depicted by light blue lines above each state marker. Transitions between electronic states often occur to higher vibrational levels which then relax to lower levels by collisional loss of heat (translational energy).
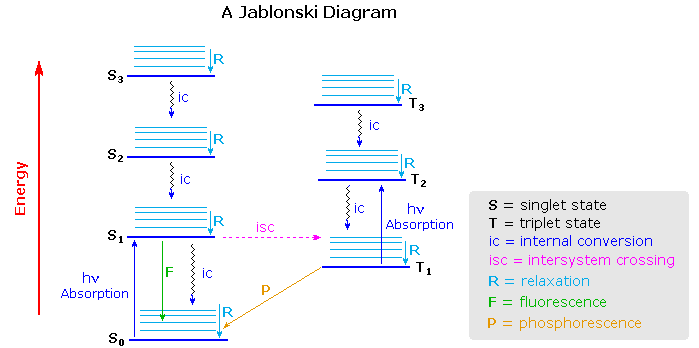
Excited states may be classified as singlet or triplet based upon their electron spin angular momentum. The electrons in most non-metallic organic compounds are paired (opposite spins) in bonding and non-bonding orbitals, resulting in a net zero spin diamagnetic molecule for the ground state. Such states have a single energy state in an applied magnetic field, and are called singlets. Electronic states in which two electrons with identical spin occupy different orbitals (the Pauli exclusion principle) have a net spin of 1 (2 • 1/2) and are paramagnetic. In a magnetic field such states have three energy levels (+1, 0, -1) and are called triplets. Molecular oxygen is a rare example of a triplet ground electronic state.
The distinction between singlet and triplet states is important because photon induced excitation always leads to a state of the same multiplicity, i.e. singlet to singlet or triplet to triplet. Since most ground states are singlets, this means that the excited states initially formed by absorption of light must also be singlets. Internal conversion of excited states to lower energy states of the same multiplicity takes place rapidly with loss of heat energy (relaxation). Alternatively, an excited state may return to the ground state by emitting a photon (radiative decay). In the study of acetone described above, nearly 80% of the excited singlet states lose energy by internal conversion and about 3% by fluorescence. Conversion of a singlet state to a lower energy triplet state, or vice versa, is termed intersystem crossing and is slower than internal conversion. Radiative decay from a triplet state is called phosphorescence and is generally quite slow. The approximate timescales for these transitions are given in the following table.
| Process | Transition | Timescale (sec) |
|---|---|---|
| Light Absorption (Excitation) | S0 → Sn | ca. 10-15 (instantaneous) |
| Internal Conversion | Sn → S1 | 10-14 to 10-11 |
| Vibrational Relaxation | Sn* → Sn | 10-12 to 10-10 |
| Intersystem Crossing | S1 → T1 | 10-11 to 10-6 |
| Fluorescence | S1 → S0 | 10-9 to 10-6 |
| Phosphorescence | T1 → S0 | 10-3 to 100 |
| Non-Radiative Decay | S1 → S0 T1 → S0 |
10-7 to 10-5 10-3 to 100 |
The non-radiative decay noted in the last row may take place by intermolecular energy transfer to a different molecule. This collisional process is termed quenching if the focus is on the initially excited species, or sensitization if the newly created excited state is of interest. Photochemical sensitization commonly occurs by a T1 + S0 → S0 + T1 reaction, where the bold red-colored species is the sensitizer. The new triplet excited state may then undergo characteristic reactions of its own.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|