


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-7-2016
Date: 17-10-2020
Date: 10-5-2019
|
Preparation of Carboxylic Acids
The carbon atom of a carboxyl group has a high oxidation state. It is not surprising, therefore, that many of the chemical reactions used for their preparation are oxidations. Such reactions have been discussed in previous sections of this text, and the following diagram summarizes most of these. To review the previous discussion of any of these reaction classes simply click on the number (1 to 4) or descriptive heading for the group.
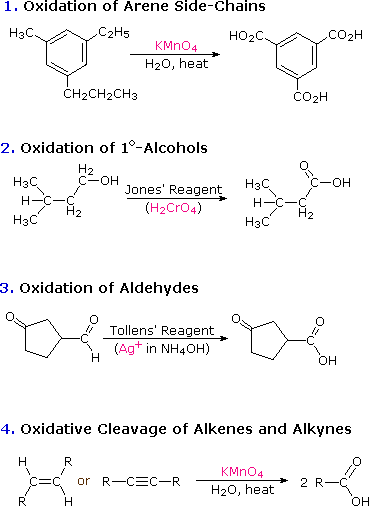
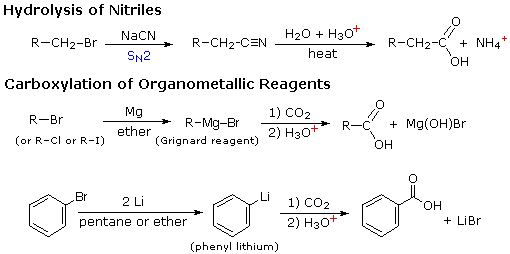
Two other useful procedures for preparing carboxylic acids involve hydrolysis of nitriles and carboxylation of organometallic intermediates. As shown in the following diagram, both methods begin with an organic halogen compound and the carboxyl group eventually replaces the halogen. Both methods require two steps, but are complementary in that the nitrile intermediate in the first procedure is generated by a SN2 reaction, in which cyanide anion is a nucleophilic precursor of the carboxyl group. The hydrolysis may be either acid or base-catalyzed, but the latter give a carboxylate salt as the initial product.
In the second procedure the electrophilic halide is first transformed into a strongly nucleophilic metal derivative, and this adds to carbon dioxide (an electrophile). The initial product is a salt of the carboxylic acid, which must then be released by treatment with strong aqueous acid.



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
"عراب الذكاء الاصطناعي" يثير القلق برؤيته حول سيطرة التكنولوجيا على البشرية ؟
|
|
|
|
|
|
|
جمعية العميد تعقد اجتماعها الأسبوعي لمناقشة مشاريعها البحثية والعلمية المستقبلية
|
|
|