

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Inductive Electronic Effects of Substituents on Acid/Base Strength
المؤلف:
ADAM RENSLO
المصدر:
the organic chemistry of medicinal agent
الجزء والصفحة:
p270
12-7-2016
4368
Inductive Electronic Effects of Substituents on Acid/Base Strength
In the above sections we examined three factors that give rise to differences in relative acidities of X–H bonds within different functional groups. Most biological and drug molecules, however, have more than one functional group (substituents) and these may influence the electronic properties and acid/base properties of each other. The extent to which the properties are altered depends on the identity and placement of the substituents relative to one another, as well as on what types of bonds connect them. In this and the following two sections we examine the three main types of interactions between substituents: (1) inductive electronic effects, (2) combined resonance and inductive electronic effects, and (3) proximity effects.
As noted for resonance electronic effects, any structural feature that withdraws or pulls electron density away from a basic X atom decreases its basicity and increases the acidity of the corresponding X–H bond. Conversely, features that donate or push electron density toward a basic X atom increase its basicity and decrease the acidity of its corresponding X–H acid. While resonance effects result from direct delocalization of electron pairs into a conjugated π system, inductive effects result from induced polarization of σ bonds connecting the basic X atom of interest and a polarized substituent. The substituent is typically a functional group with a polarized bond, that is, a dipole moment. Inductive effects can be electron donating or electron withdrawing, depending on the direction of the dipole relative to the X–H bond (Figure 1.1).

Figure 1.1 Polarized bonds between atoms with different electronegativities may be inductively electron donating or electron withdrawing. In electron donating substituents the negative end of the dipole points toward the acidic X–H group, whereas in electron withdrawing substituents the positive end of the dipole points toward the acidic X–H group.
Recall that the directionality and magnitude of the dipole in a polarized bond is determined by the relative electronegativities of the bonded atoms. Of the atoms found most often in organic molecules, only H is less electronegative than C, while all of the common heteroatoms (X = N, O, S, F, Cl, Br, I) are more electronegative than C (Table 1.1). Thus, H–C bonds are polarized with slight greater electron density on carbon and thus are electron donating. In contrast, the greater electronegativity of the X heteroatoms makes X–C bonds polarized in the opposite direction and electron withdrawing.
Recall that the directionality and magnitude of the dipole in a polarized bond is determined by the relative electronegativities of the bonded atoms. Of the atoms found most often in organic molecules, only H is less electronegative than C, while all of the common heteroatoms (X = N, O, S, F, Cl, Br, I) are more electronegative than C (Table 1.1).
Table 1.1 Electronegativities of Elements in the First Three Rows of the Periodic Table.

Thus, H–C bonds are polarized with slight greater electron density on carbon and thus are electron donating. In contrast, the greater electronegativity of the X heteroatoms makes X–C bonds polarized in the opposite direction and electron withdrawing.
As an example of the effects of the donating versus withdrawing substituents, consider the relative acidities of the O–H bond in acetic acid as compared to formic acid or chloroacetic acid (Figure 1.2). Three electron donating H–C bonds in the methyl group of acetic acid contribute electron density via σ bonds to the basic O, increasing its basicity and decreasing the acidity of the O–H bond in acetic acid (pKa ~4.8) relative to that in formic acid (pKa ~3.8). In contrast, replacement of methyl with chloromethyl reverses polarization of the substituent, which now pulls electron density away from the basic O, decreasing its basicity and increasing the acidity of the O–H bond in chloroacetic acid (pKa ~2.8) relative to that in acetic acid (pKa ~4.8).
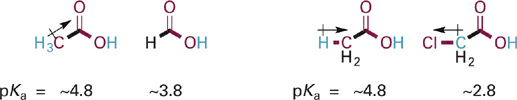
Figure 1.2 Structures and pKa values for acetic acid as compared to either formic acid (left) or chloroacetic acid (right). Dipole moments are shown for specific substituents to illustrate inductive donating or withdrawing effects. In this figure and those that follow, the σ bonds connecting the basic O atom and the substituents being compared are shown as thick bonds.
Although alkyl groups are essentially the only electron donating substituents, there are a wide variety of electron withdrawing substituents of variable strength. To a first approximation, the polarization and consequent electron withdrawing effects will increase with increasing electronegativity of X in an X–C bond. Thus, the more electronegative atom fluorine is a stronger electron withdrawing substituent than chlorine, making fluoroacetic acid a stronger acid than chloroacetic acid (Figure 1.3).
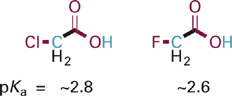
Figure 1.3 Structures and pKa values for chloroacetic acid and fluoroacetic acid.
Not surprisingly, the presence of multiple polarized X–C bonds, whether single (e.g., F3C−, Cl3C−), or double, or triple bonds (e.g., O=C−, N≡C−, O=N−), increases the polarization and consequent inductive withdrawing effect of a substituent (Figure 1.4). Note that the nitrile (N≡C–) and nitro groups (NO2) in these examples exert their withdrawing effects via an inductive effect, since the basic O atom is not conjugated with the π bonds of these substituents.
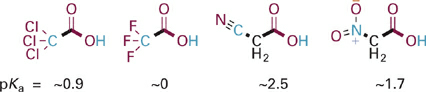
Figure 1.4 Examples of increased inductive electron withdrawing effect of substituents with multiple X–C bonds on the acidity of the O–H bond.
Groups with double and triple bonds between C atoms (C=C or C≡C), although not polar themselves, are electron deficient and thus weakly pull electron density toward them through connecting σ bonds. This effect is evident in the case of the phenyl substituent in benzoic acid, which reduces the pKa slightly as compared to acetic acid. In contrast to the dramatic resonance effects observed in the cases of aniline and phenol, the effect in benzoic acid is more subtle and purely inductive since the aryl ring is connected to the basic O atom by two σ bonds rather than being conjugated with it (Figure 1.5).
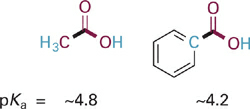
Figure 1.5 Structures and pKa values for acetic acid and benzoic acid. The phenyl ring in the latter is weakly electron withdrawing by an inductive effect.
In the remainder of this section we will examine how the ionization state (charge state) of functional groups can dramatically affect their inductive withdrawing or donating effects. This phenomenon is highly relevant for proteins, and especially enzyme active sites where a number of ionizable groups are usually found in close proximity. Here we will observe these effects in the context of small molecules with multiple ionizable groups.
First, we consider the carboxylic acid functional group (COOH) and its ionized (anionic) form, the carboxylate anion (COO−). To evaluate the inductive effect of COOH, we can compare the pKa values of acetic acid with the dicarboxylic acids, succinic acid, and malonic acid (Figure 1.6). In the fully protonated diacids, theindistinguishable COOH groups are electron withdrawing, which we can see by noting that the lowest pKa value for either of the diacids is lower (more acidic) than acetic acid. However, once a proton is lost from a diacid the negatively charged carboxylate anion COO− exerts an electron donating effect on the remaining COOH group, decreasing its acidity substantially (higher second pKa compared to acetic acid). Another thing to note is that the inductive withdrawing effect of COOH in the fully protonated diacids exhibits much stronger distance dependence than does the donating effect of the carboxylate anion COO−. The inductive withdrawing effect of the COOH group is akin to a dipole-dipole interaction and thus stronger in malonic acid than in succinic acid. By contrast, the effect of the carboxylate anion in the second deprotonation step can be thought of as an unfavorable charge-charge interaction.
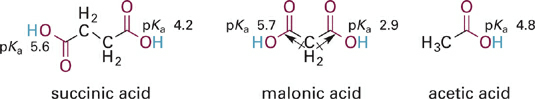
Figure 1.6 Comparison of the acidity of acetic acid with two related dicarboxylic acids. The uncharged COOH groups act as inductive electron withdrawing groups (dipole arrows shown on malonic acid), but the negatively charged COO− group is inductively electron donating. The result of this is that the first pKa of the diacids is lower than for acetic acid while the second pKa is higher.
In contrast to the opposing effects of COOH and COO−, charged ammonium ions (−NR3+) and neutral amines (−NR2) are both electron withdrawing, but the charged forms are much more strongly withdrawing. These effects are apparent in comparing the acidities of two diammonium compounds with that of ethylammonium ion (Figure 1.7). The withdrawing effect of the ammonium group lowers the first pKa for the diacids relative to ethylamine, an effect that can be viewed as a combination of a strong inductive withdrawing effect and a repulsive ionic interaction present only in the doubly charged forms (and which is relieved by deprotonation). After the first proton is lost, the resulting neutral amino group retains a weakly withdrawing effect as evidenced by the greater acidity of the second ammonium group in 1,2,-diaminoethane (pKa = 9.9) as compared to ethylamine (pKa = 10.6). In 1,3-diaminopropane, however, the inductive withdrawing effect is not significantly transmitted over the four σ bonds separating the amino groups, so the second pKa value is only comparable to that of ethylamine.
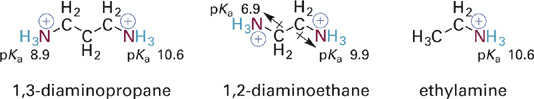
Figure 1.7 Comparison of acidities of diammonium ions relative to the mono-ammonium ion of ethylamine.
The distance dependence of inductive electronic effects exemplified above with charged substituents can also be demonstrated by systematically moving a polarized bond further away from a basic atom X (Figure 1.8). In the mono-chlorobutanoic acids shown below, the inductive effect of the polarized C−Cl bond is strongest in chloroacetic acid (pKa = 2.8) and accordingly weaker as additional σ bonds are introduced between the withdrawing substituent and the basic O atom of the COOH function. Note that with just two σ bonds intervening, the withdrawing effect of the C−Cl bond is reduced significantly (pKa = 4.1), and with three σ bonds intervening, the effect is nearly eliminated (pKa = 4.5 vs. 4.8 for acetic acid).

Figure 1.8 The distance dependence of inductive effects is illustrated with a series of carboxylic acids bearing a polarized C–Cl bond at various distances from the carboxylic acid. Acetic acid (far right) serves as a reference compound lacking the withdrawing substituent.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















