


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-9-2019
Date: 22-12-2016
Date: 29-10-2020
|
Benzene and Other Aromatic Compounds
The adjective "aromatic" is used by organic chemists in a rather different way than it is normally applied. It has its origin in the observation that certain natural substances, such as cinnamon bark, wintergreen leaves, vanilla beans and anise seeds, contained fragrant compounds having common but unexpected properties. Cinnamon bark, for example, yielded a pleasant smelling compound, formula C9H8O, named cinnamaldehyde. Because of the low hydrogen to carbon ratio in this and other aromatic compounds (note that the H:C ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Since double bonds are easily cleaved by oxidative reagents such as potassium permanganate or ozone, and rapidly add bromine and chlorine, these reactions were applied to these aromatic compounds. Surprisingly, products that appeared to retain many of the double bonds were obtained, and these compounds exhibited a high degree of chemical stability compared with known alkenes and cycloalkenes (aliphatic compounds). On treatment with hot permanganate solution, cinnamaldehyde gave a stable, crystalline C7H6O2 compound, now called benzoic acid. The H:C ratio in benzoic acid is <1, again suggesting the presence of several double bonds. Benzoic acid was eventually converted to the stable hydrocarbon benzene, C6H6, which also proved unreactive to common double bond transformations, as shown below. For comparison, reactions of cyclohexene, a typical alkene, with these reagents are also shown (green box). As experimental evidence for a wide assortment of compounds was acquired, those incorporating this exceptionally stable six-carbon core came to be called "aromatic".

If benzene is forced to react by increasing the temperature and/or by addition of a catalyst, It undergoes substitution reactions rather than the addition reactions that are typical of alkenes. This further confirms the previous indication that the six-carbon benzene core is unusually stable to chemical modification. The conceptual contradiction presented by a high degree of unsaturation (low H:C ratio) and high chemical stability for benzene and related compounds remained an unsolved puzzle for many years. Eventually, the presently accepted structure of a regular-hexagonal, planar ring of carbons was adopted, and the exceptional thermodynamic and chemical stability of this system was attributed to resonance stabilization of a conjugated cyclictriene.
Benzene: 

Here, two structurally and energetically equivalent electronic structures for a stable compound are written, but no single structure provides an accurate or even an adequate representation of the true molecule. The six-membered ring in benzene is a perfect hexagon (all carbon-carbon bonds have an identical length of 1.40 Å). The cyclohexatriene contributors would be expected to show alternating bond lengths, the double bonds being shorter (1.34 Å) than the single bonds (1.54 Å). An alternative representation for benzene (circle within a hexagon) emphasizes the pi-electron delocalization in this molecule, and has the advantage of being a single diagram. In cases such as these, the electron delocalization described by resonance enhances the stability of the molecules, and compounds composed of such molecules often show exceptional stability and related properties.
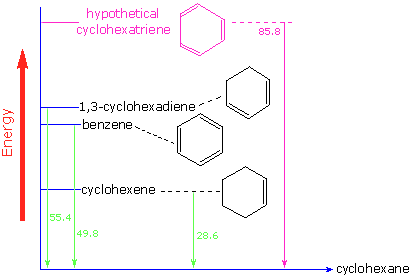
Evidence for the enhanced thermodynamic stability of benzene was obtained from measurements of the heat released when double bonds in a six-carbon ring are hydrogenated (hydrogen is added catalytically) to give cyclohexane as a common product. In the following diagram cyclohexane represents a low-energy reference point. Addition of hydrogen to cyclohexene produces cyclohexane and releases heat amounting to 28.6 kcal per mole. If we take this value to represent the energy cost of introducing one double bond into a six-carbon ring, we would expect a cyclohexadiene to release 57.2 kcal per mole on complete hydrogenation, and 1,3,5-cyclohexatriene to release 85.8 kcal per mole. These heats of hydrogenation would reflect the relative thermodynamic stability of the compounds. In practice, 1,3-cyclohexadiene is slightly more stable than expected, by about 2 kcal, presumably due to conjugation of the double bonds. Benzene, however, is an extraordinary 36 kcal/mole more stable than expected. This sort of stability enhancement is now accepted as a characteristic of all aromatic compounds.
A molecular orbital description of benzene provides a more satisfying and more general treatment of "aromaticity". We know that benzene has a planar hexagonal structure in which all the carbon atoms are sp2 hybridized, and all the carbon-carbon bonds are equal in length. As shown below, the remaining cyclic array of six p-orbitals ( one on each carbon) overlap to generate six molecular orbitals, three bonding and three antibonding. The plus and minus signs shown in the diagram do not represent electrostatic charge, but refer to phase signs in the equations that describe these orbitals (in the diagram the phases are also color coded). When the phases correspond, the orbitals overlap to generate a common region of like phase, with those orbitals having the greatest overlap (e.g. π1) being lowest in energy. The remaining carbon valence electrons then occupy these molecular orbitals in pairs, resulting in a fully occupied (6 electrons) set of bonding molecular orbitals. It is this completely filled set of bonding orbitals, or closed shell, that gives the benzene ring its thermodynamic and chemical stability, just as a filled valence shell octet confers stability on the inert gases.

The Molecular Orbitals of Benzene



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|