

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Immunoelectrophoresis
المؤلف:
Clive Dennison
المصدر:
A guide to protein isolation
الجزء والصفحة:
20-4-2016
3501
Immunoelectrophoresis
Immunoelectrophoretic methods combine electrophoresis with subsequent immuno-detection, and the conditions used are a compromise of the optimum for each of the two steps. A number of immunoelectrophoresis methods will be discussed below, as it is useful to have an appreciation of the principles of each method, but it must be emphasized that methods involving immunoprecipitation have today largely been replaced by methods involving amplification, most commonly with enzymes.
1. Cross-over electrophotesis
One of the limitations of the Ouchterlony double diffusion system is that a large proportion of the Ab and Ag diffuse in non-productive directions and are thus wasted. Only that proportion of the Ab that diffuses towards the Ag, and vice versa, will productively form immunoprecipitate.
It will be recalled from the discussion of paper electrophoresis that due to Electroendosmosis the γ-globulin which are the antibodies migrate towards the cathode, whereas all of the other serum proteins migrate towards the anode. For the analysis of blood proteins, this provides a way of ensuring that all of the Ag meets all of the Ab, by a process known as cross-over electrophoresis. In this process, the Ab and Ag are placed in two wells in an agarose gel and subjected to electrophoresis as illustrated in Fig. 1. In this way all of the Ag encounters all of the Ab and wasteful diffusion is obviated. This may be useful when the amount of Ag available is limited, for example in forensics.

Figure 1. Cross-over electrophoresis.
2. Rocket electrophoresis
A limitation of Mancini radial diffusion is that differences in the diameters of the precipitin rings may be small. The reason for this is that diffusion is in all directions, and a consequence is reduced sensitivity In the “rocket” electrophoresis method of Laure, diffusion of the Ag is replaced by electrophoresis of the Ag into the gel containing the Ab. Electrophoretic migration of the antigen occurs in one direction only so that immunoprecipitation, instead of occurring in a circle. occurs in a rocket shape (Fig. 2). hence the name.

Figure 2. Rocket electrophoresis.
An advantage of rocket electrophoresis is that it is more sensitive than Mancini radial diffusion. because, being “pulled out” in one direction only, differences in the length of the rockets are greater and are more easily measured. A standard curve of rocket length vs antigen concentration can be constructed and used to determine the concentration of unknowns analyzed under the same conditions.
3. Grabar-Williams immunoelectrophoresis
Grabar-Williams immunoelectrophoresis is a binary method in which an Ag mixture is first separated by electrophoresis and the separated components are subsequently detected by immuno-diffusion. It may be considered as a development of the Ouchterlony technique, with better resolving power because of the separation of the Ag mixture in the electrophoresis step.
Conditions used have to be a compromise between the requirements of the two stages. For example;-
• Electrophoresis is best performed in a sieving gel, such as polyacrylamide, partly because this restricts diffusion. However, immunodiffusion is dependent upon diffusion and so agarose, a non-sieving gel, is used as this does not impede diffusion.
• The requirement for immunoprecipitation restricts the buffer pH, which must not be too far from physiological pH.
• Both electrophoresis and immunoprecipitation require a buffer of low ionic strength.
The sample is introduced into a well cut into an agarose gel, supported on a glass slide, and is separated by electrophoresis. To prevent protein from migrating off the end of the gel, a sample of bromophenol blue may
be run in parallel with the protein and the run stopped when the bromophenol blue reaches the end of the gel. A trough is then cut, parallel to the direction of electrophoresis and a few millimeters from the well (Fig. 3). Ab is introduced into the trough and the gel is incubated as described for the Ouchterlony technique for several days to allow formation of the immunoprecipitin bands.
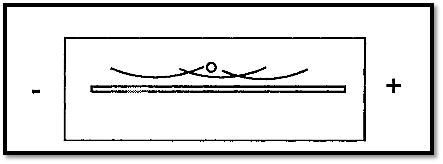
Figure 3. Grabar-Williams immunoelectrophoresis.
Optimal proportions of Ab and Ag may be established by a prior Ouchterlony analysis, or replicate runs, covering a range of Ab and Ag concentrations, may be carried out.
4. Clarke-Freeman 2-D immunoelectrophoresis
Rocket electrophoresis is a method for the quantitation of Ags by electrophoresis into an Ab-containing gel. In the rocket method different Ags are placed in different wells and either the Ag must be pure or, more usually, a mono-specific antiserum is used. In Grabar-Williams immunoelectrophoresis impure Ag mixtures are first separated by electrophoresis, before being analyzed by immuno-precipitation. The Clarke-Freeman technique combines aspects of Grabar-Williams immunoelectrophoresis and of rocket electrophoresis. The sample is first separated by electrophoresis in one dimension. as in the Grabar-Williams technique, and then at right-angles to the first electrophoresis is electrophoretically drawn into an Ab-containing gel, as in the rocket technique. Like rocket electrophoresis, the latter stage is a form of cross-over electrophoresis and so an advantage over the Grabar-Williams technique is that all of the Ag meets Ab and there is no wasteful, unproductive, diffusion.

Figure 4. Clarke-Freeman immunoelectrophoresis.
Sample in the well is separated by electrophoresis in the first dimension. A gel containing Ab is then cast adjoining the first gel along one of its edges. The separated sample components are then drawn into the Ab-containing gel by electrophoresis in the second dimension.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















