

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Distribution of Hydrophobic Ionogenic Organic Compounds
المؤلف:
SOMENATH MITRA
المصدر:
Sample Preparation Techniques in Analytical Chemistry
الجزء والصفحة:
p 57
2-3-2018
2426
Distribution of Hydrophobic Ionogenic Organic Compounds
Some highly hydrophobic weak acids and bases exhibit substantial hydrophobicity even in the ionized state. For highly hydrophobic ionogenic organic compounds, not only is transfer of the neutral species between the aqueous phase and the immiscible phase important, but the transfer of the hydrophobic, ionized, organic species as free ions or ion pairs may also be significant. Mathematically, this is described by refining then-octanol/ water partition coefficient to reflect the pHdependent distribution between water (W) andn octanol (O) of chemical X in both the ionized and nonionized forms. If chemical X is a weak acid, HA, the distribution ratio is
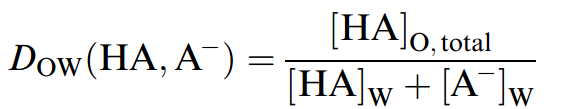
where [HA]O;total is the sum of all neutral species, free ions, and ions paired with inorganic counterions that transfer to octanol.
For example, the ratio of then-octanol/water distribution coefficient of the nondissociated species to that of the ionic species is nearly 10,000 for 3-methyl-2-nitrophenol, but only about 1000 for pentachlorophenol because of the greater significance of the hydrophobicity of the ionized form of pentachlorophenol. The logarithm of then-octanol/water distribution coefficient of pentachlorophenol as the phenolate is about 2 (determined at pH 12, and 0.1MKCl), which indicates significant distribution of the ionized form into then-octanol phase. Extraction of such highly hydrophobic ionogenic organic compounds can result from mixed-mode mechanisms that incorporate both the hydrophobic and ionic character of the compound.
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















