


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-2-2018
Date: 25-6-2020
Date: 7-7-2020
|
Arsenic, antimony and bismuth
Arsenic vapour contains As4 molecules, and the unstable yellow form of solid As probably also contains these units; at relatively low temperatures, Sb vapour contains molecular Sb4. At room temperature and pressure, As, Sb and Bi are grey solids with lattice structures resembling that of black phosphorus (Figure 1.1c).
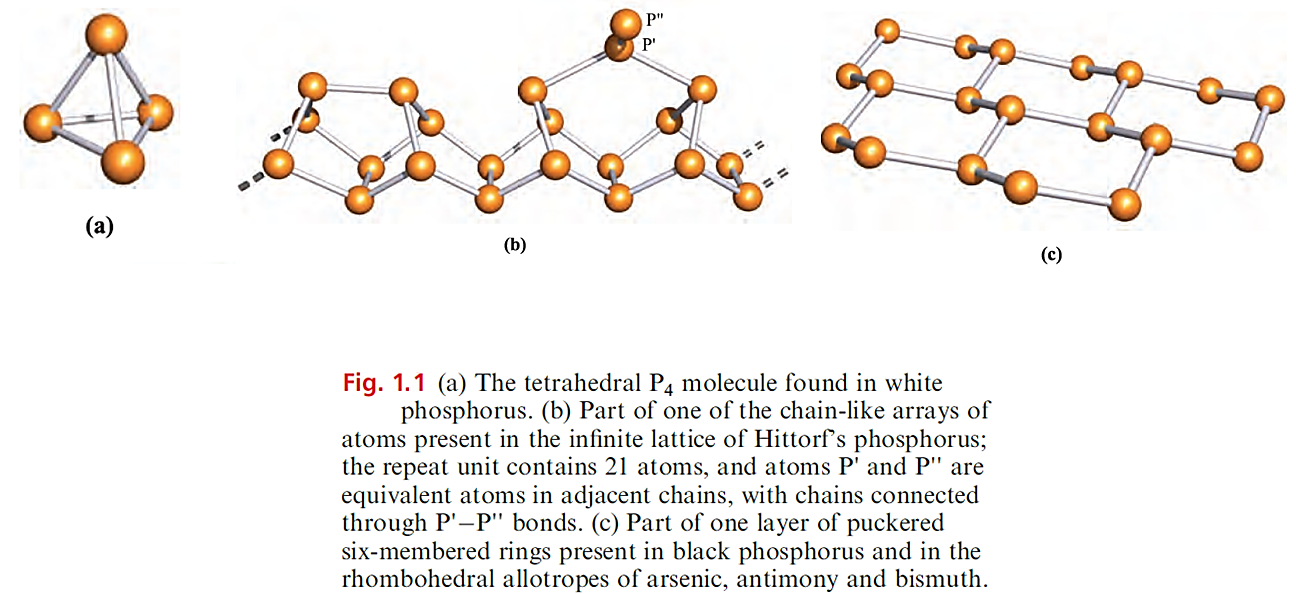
On descending the group, although intralayer bond distances increase as expected, similar increases in interlayer spacing do not occur, and the coordination number of each atom effectively changes from 3 (Figure 1.1c) to 6 (three atoms within a layer and three in the next layer). Arsenic, antimony and bismuth burn in air and combine with halogens.
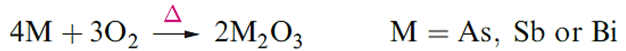
They are not attacked by non-oxidizing acids but react with concentrated HNO3 to give H3AsO4 (hydrated As2O5), hydrated Sb2O5 and Bi(NO3)3 respectively, and with concentrated H2SO4 to produce As4O6, Sb2 (SO4)3 and Bi2(SO4)3 respectively. None of the elements reacts with aqueous alkali, but As is attacked by fused NaOH.
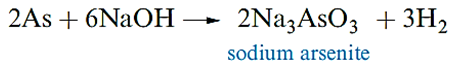



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|