


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-6-2020
Date: 9-1-2017
Date: 19-7-2020
|
Phosphorus
Phosphorus exhibits complicated allotropy; eleven forms have been reported, of which at least five are crystalline. Crystalline white phosphorus contains tetrahedral P4 molecules (Figure 1.1a) in which the P_P distances (221pm) are consistent with single bonds (rcov = 110 pm). White phosphorus is defined as the standard state of the element, but is actually metastable.
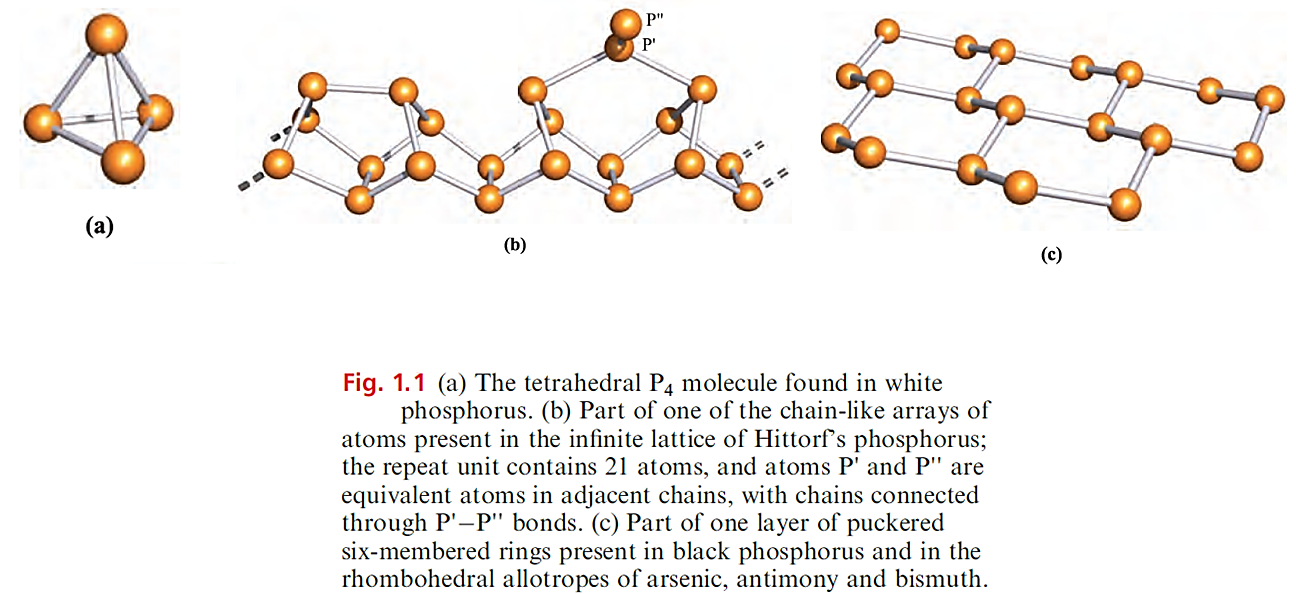
The lower stability of the white form probably originates from strain associated with the 608 bond angles.
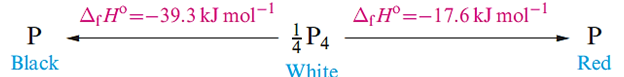
White phosphorus is manufactured , and heating this allotrope in an inert atmosphere at ≈ 540K produces red phosphorus. Several crystalline forms of red phosphorus exist, and all probably possess infinite lattices. Hittorf’s phosphorus (also called violet phosphorus) is a well-characterized form of the red allotrope and its complicated structure is best described in terms of interlocking chains (Figure 1.1b). Non-bonded chains lie parallel to each other to give layers, and the chains in one layer lie at rightangles to the chains in the next layer, being connected by the P`_P`` bonds shown in Figure 1.1b. All P_P bond distances are ≈ 222 pm, indicating covalent single bonds.
Black phosphorus is the most stable allotrope and is obtained by heating white phosphorus under high pressure. Its appearance and electrical conductivity resemble those of graphite, and it possesses a double-layer lattice of puckered 6-membered rings (Figure 1.1c); P_P distances within a layer are 220pm and the shortest interlayer P_P distance is 390 pm. On melting, all allotropes give a liquid containing P4 molecules, and these are also present in the vapour; above 1070K or at high pressures, P4 is in equilibrium with P2.
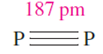
Most of the chemical differences between the allotropes of phosphorus are due to differences in activation energies for reactions. Black phosphorus is kinetically inert and does not ignite in air even at 670 K. Red phosphorus is intermediate in reactivity between the white and black allotropes. It is not poisonous, is insoluble in organic solvents, does not react with aqueous alkali, and ignites in air above 520 K. It reacts with halogens, sulfur and metals, but less vigorously than does white phosphorus. The latter is a soft, waxy solid which becomes yellow on exposure to light; it is very poisonous, being readily absorbed into the blood and liver. White phosphorus is soluble in benzene, PCl3 and CS2 but is virtually insoluble in water, and is stored under water to prevent oxidation. In moist air, it undergoes chemiluminescent oxidation, emitting a green glow and slowly forming P4O8 and some O3; the chain reaction involved is extremely complicated.
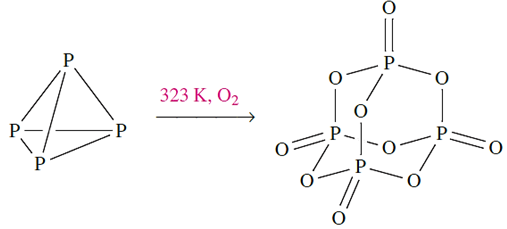
Above 323 K, white phosphorus inflames, yielding phosphorus(V) oxide (equation 14.8); in a limited supply of air, P4O6 may form. White phosphorus combines violently with all of the halogens giving PX3 (X=F, Cl, Br, I) or PX5 (X=F, Cl, Br) depending on the relative amounts of P4 and X2.
Concentrated HNO3 oxidizes P4 to H3PO4, and with hot aqueous NaOH, reaction 14.9 occurs, some H2 and P2H4 also being formed.
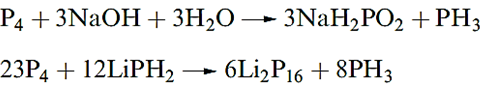
Reaction above yields Li2P16, while Li3P21 and Li4P26 can be obtained by altering the ratio of P4 :LiPH2. The structures ofvthe phosphide ions [P16]2-, 14.9, [P21]3- and [P26]4- are related to one chain in Hittorf’s phosphorus (Figure 1.1b).

Like N2, P4 can act as a ligand in d-block metal complexes. Examples of different coordination modes of P4 are shown in structures below.
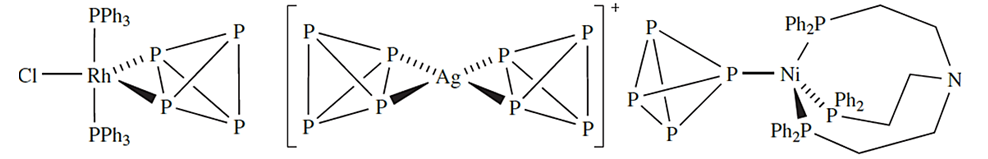



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|