


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-4-2019
Date: 9-4-2017
Date: 4-7-2017
|
Silicones
Although silicones are organometallic compounds, they are conveniently described in this chapter because of their structural similarities to silicates. Hydrolysis of MenSiCl4-n (n = 1–3) might be expected to give the derivatives MenSi(OH)4-n (n = 1–3). By analogy with carbon analogues, we might expect Me3SiOH to be stable (except with respect to dehydration at higher temperatures), but Me2Si(OH)2 and MeSi(OH)3 undergo dehydration to Me2Si=O and MeSiO2H respectively. However we indicated that an Si=O bond is energetically less favourable than two Si_O bonds. As a consequence, hydrolysis of MenSiCl4-n (n = 1–3) yields silicones which are oligomeric products containing the tetrahedral groups in which each O atom represents part of an Si_O_Si bridge. Diols can condense to give chains or rings. Hydrolysis of MeSiCl3 produces a cross-linked polymer.
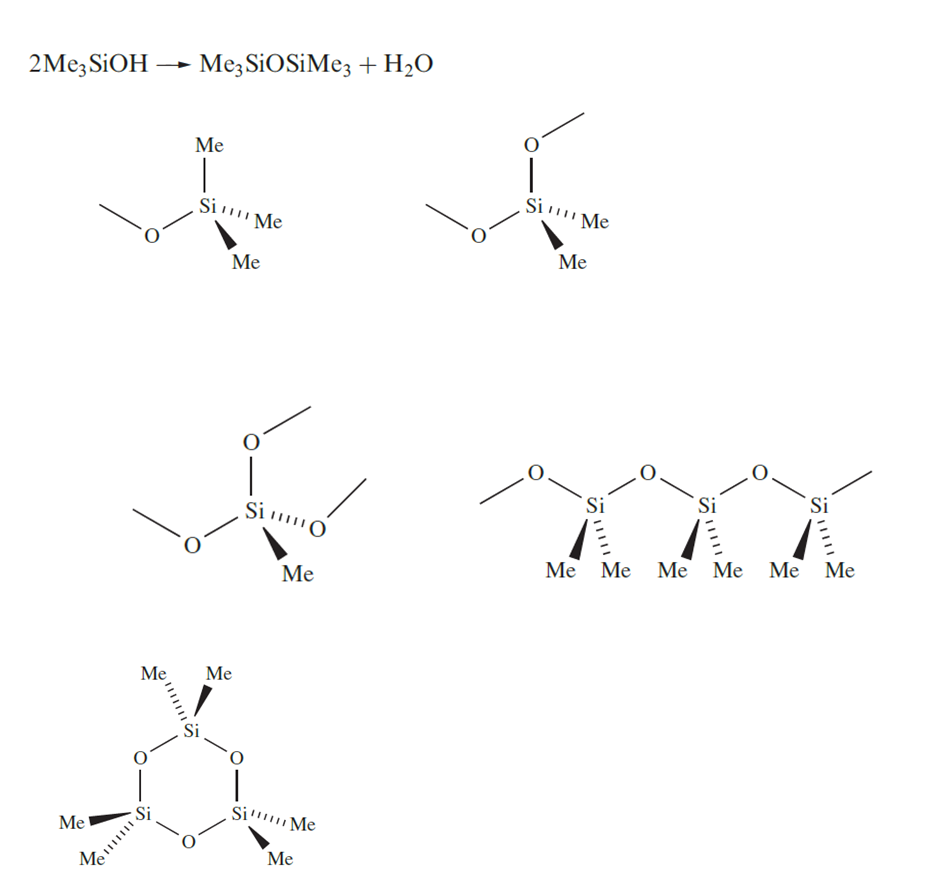
Silicone polymers have a range of structures and applications and, in their manufacture, control of the polymerization is essential. The methylsilicon chlorides are co-hydrolysed, or the initial products of hydrolysis are equilibrated by heating with H2SO4 which catalyses the conversion of cyclic oligomers into chain polymers, bringing about redistribution of the terminal OSiMe3 groups. For example, equilibration of HOSiMe2(OSiMe2)nOSiMe2OH with Me3SiOSiMe3 leads to the polymer Me3Si(OSiMe2)nOSiMe3. Cross-linking, achieved by cohydrolysis of Me2SiCl2 and MeSiCl3, leads, after heating at 520 K, to silicone resins that are hard and inert; tailoring the product so that it possesses a smaller degree of crosslinking results in the formation of silicone rubbers.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|