
Fullerenes: synthesis and structure
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Inorganic Chemistry
المصدر:
Inorganic Chemistry
 الجزء والصفحة:
p 348
الجزء والصفحة:
p 348
 2-2-2018
2-2-2018
 2116
2116
Fullerenes: synthesis and structure
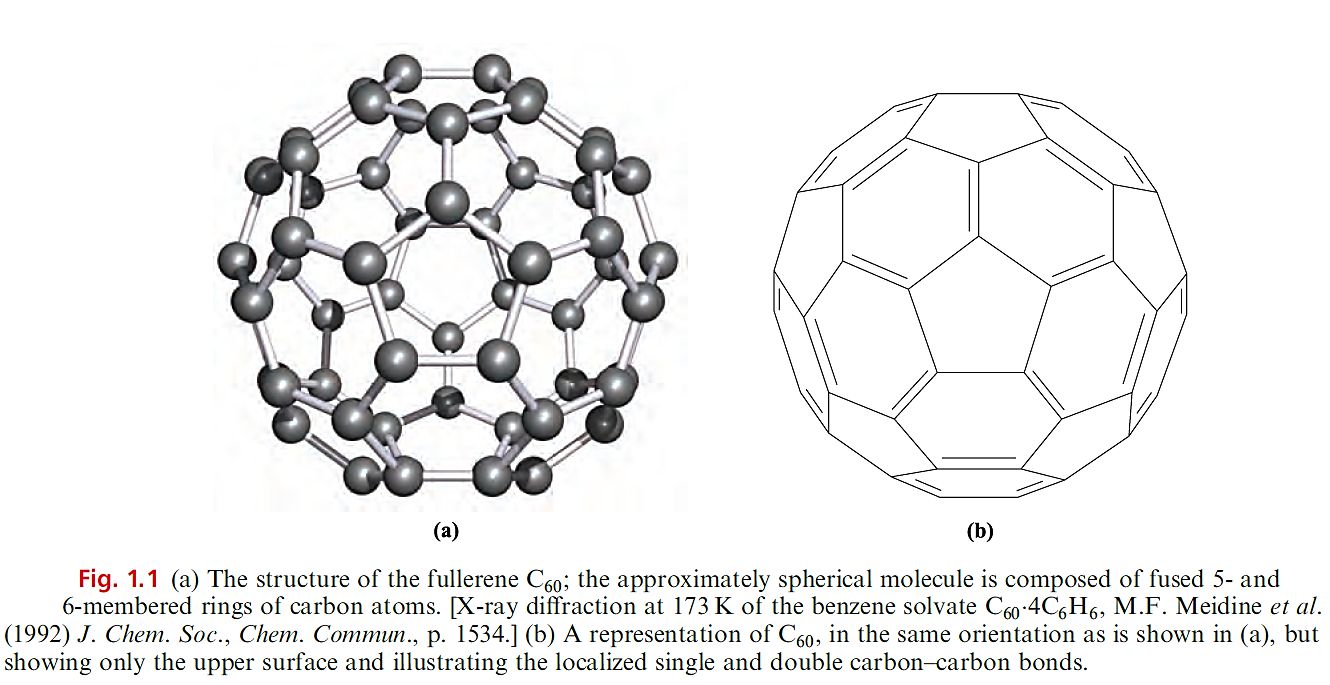
In 1985, Kroto, Smalley and coworkers discovered that, by subjecting graphite to laser radiation at >10 000 K, new allotropes of carbon were formed. The fullerenes are named after architect Buckminster Fuller, known for designing geodesic domes. Each fullerene is molecular and the family includes C60, C70, C76, C78, C80 and C84. Several synthetic routes to fullerenes have been developed; C60 and C70 are the major components of the mixture formed when graphitic soot is produced as graphite rods are evaporated (by applying an electrical arc between them) in a helium atmosphere at ≈130 bar and the vapour condensed. Extraction of the soot into benzene yields a red solution from which C60 and C70 can be separated by chromatography. Hexane or benzene solutions of C60 are magenta, while those of C70 are red. Both C60 and C70 are now available commercially, and this has encouraged rapid exploration of their chemical properties. Figure 1.1a shows the structure of C60. Although a number of X-ray diffraction studies of C60 have been carried out, the near-spherical shape of the molecule has led to frustrating orientational disorder problems. The C60 molecule belongs to the Ih point group and consists of an approximately spherical network of atoms which are connected in 5- and 6-membered rings; all the C atoms are equivalent, as indicated by the fact that the 13C NMR spectrum of C60 exhibits one signal (δ + 143). The rings are arranged such that no 5-membered rings are adjacent to each other. Thus, C60 (the smallest fullerene that can be isolated as a stable species) satisfies the Isolated Pentagon Rule (IPR).† The separation of the 5-membered rings by 6-membered rings is easily seen in the schematic representation of C60 shown in Figure 1.1b which also gives a bonding scheme. Each C atom is covalently bonded to three others in an approximately trigonal planar arrangement; the relatively large surface of the ‘sphere’ means that there is only slight deviation from planarity at each C centre. There are two types of C_C bond: those at the junctions of two hexagonal rings (6,6- edges) are of length 139 pm, while those between a hexagonal and a pentagonal ring (5,6-edges) are longer, 145.5 pm. These differences indicate the presence of localized double and single bonds, and similar bonding descriptions are appropriate for other fullerene cages. We consider chemical evidence for the presence of C=C double bonds below. After C60, the next smallest fullerene to satisfy the IPR is C70. The C70 molecule has D5h symmetry and is approximately ellipsoidal it comprises 6- and 5-membered rings organized so that, as in C60, 5-membered rings are never adjacent. The 13C NMR spectrum of C70 confirms that there are five C environments in solution, consistent with the solid state structure.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


