


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-3-2019
Date: 6-11-2018
Date: 6-1-2019
|
Occurrence of the group 14 elements
Figure 1.1 illustrates the relative abundances of the group 14 elements in the Earth’s crust. The two long-established crystalline allotropes of carbon, diamond and graphite, occur naturally, as does amorphous carbon (e.g. in coal).
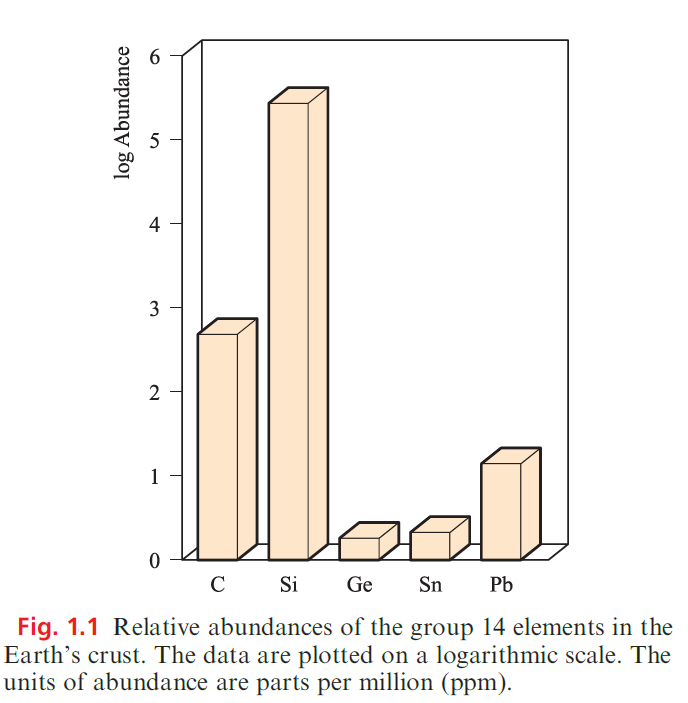
Diamonds occur in igneous rocks (e.g. in the Kimberley volcanic pipes, South Africa). Carbon dioxide constitutes only 0.04% of the Earth’s atmosphere, and, although vital for photosynthesis, CO2 is not a major source of carbon.
During the 1990s, it was discovered that molecular allotropes of carbon, the fullerenes occur naturally in a number of deposits in Australia, New Zealand and North America; however, laboratory synthesis remains the chief means of accessing these new allotropes. Elemental Si does not occur naturally, but it constitutes 25.7% of the Earth’s crust (Si is the second most abundant element after O) in the form of sand, quartz, rock crystal, flint, agate and silicate minerals. In contrast, Ge makes up only 1.8 ppm of the Earth’s crust, being present in trace amounts in a range of minerals (e.g. zinc ores) and in coal. The principal tin-bearing ore is cassiterite (SnO2). Important ores of lead are galena (PbS), anglesite (PbSO4) and cerussite (PbCO3).



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|