


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-11-2018
Date: 28-12-2018
Date: 4-11-2020
|
Redox reactions in aqueous solution
The standard reduction potentials for the M3+/M couples (Table 1.1) show that Al3+(aq) is much less readily reduced in aqueous solution than are the later M3+ions.

This can be attributed, in part, to the more negative Gibbs energy of hydration of the smaller Al3+ ion, but an important contributing factor in differentiating between the values of Eo for the Al3+/Al and Ga3+/Ga couples is the significant increase in the sum of the first three ionization energies (Table 1.1).
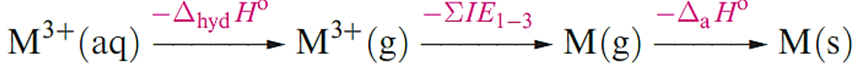
Although In(I) can be obtained in low concentration by oxidation of an In anode in dilute HClO4, the solution rapidly evolves H2 and forms In(III). A value of _0.44V has been measured for the In3+/In couple (equation 12.69).

For the Ga3+(aq)/Ga(aq) couple, a value of Eo = _0:75V has been determined and, therefore, studies of aqueous Ga are rare because of the ease of oxidation of Ga to Ga3+. The compound Ga[GaCl4]- can be used as a source of Ga in aqueous solution, but it is very unstable and rapidly reduces [I3]-, aqueous Br2, [Fe(CN)6]3- and [Fe(bpy)3]3+.
Eo for the reduction of Tl(III) to Tl(I) in molar HClO4 is 1.25 V, and under these conditions, Tl(III) is a powerful oxidizing agent. The value of Eo is, however, dependent on the anion present and complex formed (see Section 7.3); Tl(I) (like the alkali metal ions) forms few stable complexes in aqueous solution, whereas Tl(III) is strongly complexed by a variety of anions. For example, consider the presence of Cl- in solution. Whereas TlCl is fairly insoluble, Tl(III) formsthesolublecomplex [TlCl4]-and, at [Cl]- = 1mol dm_3, Eo(Tl3+=Tl+( = 0.9 V. Thallium(III) forms a more stable complex with I- than Cl-, and at high [I]-, [TlI4]- is produced in solution even though Eo(Tl3+=Tl+) is more positive than Eo(I2=2I-) (0.54 V) and TlI is sparingly soluble. Thus, while tabulated reduction potentials for the Tl3+=Tl+ and I2=2I- couples might suggest that aqueous I- will reduce Tl(III) to Tl(I) , in the presence of high concentrations of I- ,Tl(III) is stabilized.
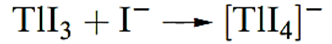
In alkaline media, Tl(I) is also easily oxidized, since TlOH is soluble in water and hydrated Tl2O3 (which is in equilibrium with Tl3+and [OH]- ions in solution) is very sparingly soluble in water (Ksp ≈ 10 -45).



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|