

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Coordination complexes of the M3+ ions
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 324
1-2-2018
1994
Coordination complexes of the M3+ ions
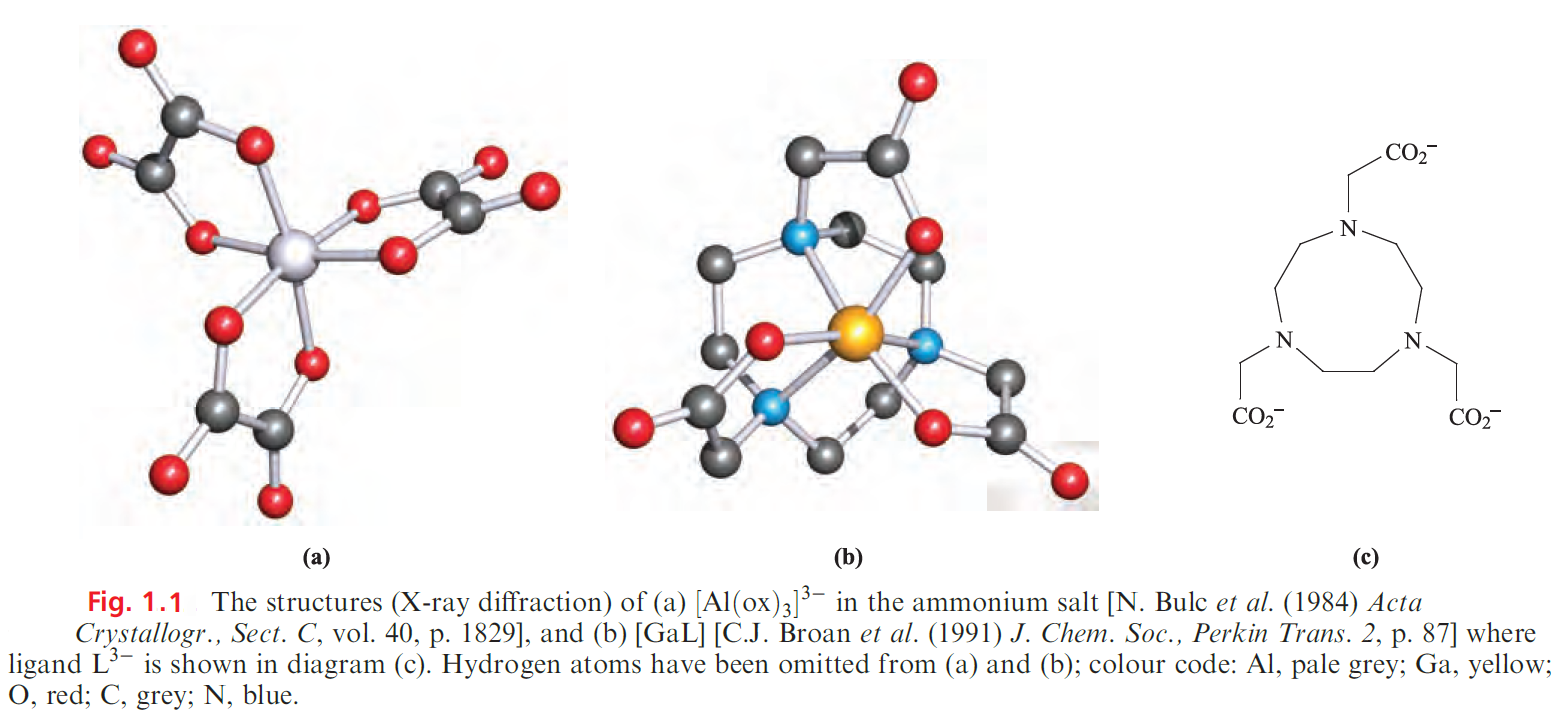
Increasing numbers of coordination complexes of the group 13 metal ions are now known. Octahedral coordination is common, e.g. in [M(acac)3] (M = Al, Ga, In), [M(ox)3]3- (M = Al, Ga, In) and mer-[Ga(N3)3(py)3]. Figure 1.1a shows the structure of [Al(ox)3]3-. The complexes[M(acac)3] are structurally related to [Fe(acac)3] so we discussed the influence of [H]+ on the formation of [Fe(acac)3] and similar arguments apply to the group 13 metal ion complexes.

Deprotonation of 8-hydroxyquinoline gives the didentate ligand which has a number of applications. For example, Al3+ may be extracted into organic solvents as the octahedral complex [Al(8-hydroxyquinoline)3] providing a weighable form of Al3+ for the gravimetric analysis of aluminium.
Complexes involving macrocyclic ligands with pendant carboxylate or phosphate groups have received attention in the development of highly stable metal complexes suitable for in vivo applications, e.g. tumour-seeking complexes containing radioisotopes . The incorporation of 67Ga (gemitter, t1/2 = 3.2 days), 68Ga ( β+-emitter, t1/2 = 68 min) or 111 In (g-emitter, t1/2 = 2.8 days) into such complexes yields potential radiopharmaceuticals. Figure 1.1c shows an example of a well-studied ligand which forms very stable complexes with Ga(III) and In(III) (logK ≥ 20). The way in which this ligand encapsulates the M3+ ion with the three N-donor atoms forced into a fac-arrangement can be seen in Figure 1.1b.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















