


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-5-2019
Date: 5-5-2019
Date: 5-2-2018
|
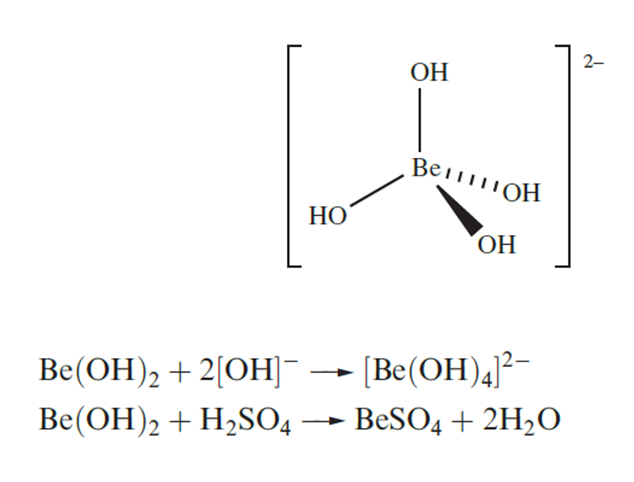
Hydroxides of e group 2 metals
Beryllium hydroxide is amphoteric and this sets it apart from the hydroxides of the other group 2 metals which are basic. In the presence of excess [OH]-, Be(OH)2 behaves as a Lewis acid forming the tetrahedral complex ion but Be(OH)2 also reacts with acids.
The water solubilities of M(OH)2 (M = Mg, Ca, Sr, Ba) increase down the group, as do their thermal stabilities with respect to decomposition into MO and H2O. Magnesium hydroxide acts as a weak base, whereas Ca(OH)2, Sr(OH)2 and Ba(OH)2 are strong bases. Soda lime is a mixture of NaOH and Ca(OH)2 and is manufactured from CaO and aqueous NaOH; it is easier to handle than NaOH and is commercially available, being used, for example, as an absorbent for CO2, and in qualitative tests for [NH4]+ salts, amides, imides and related compounds which evolve NH3 when heated with soda lime.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|