

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-4-2019
Date: 26-2-2019
Date: 5-5-2019
|
Non-aqueous coordination chemistry
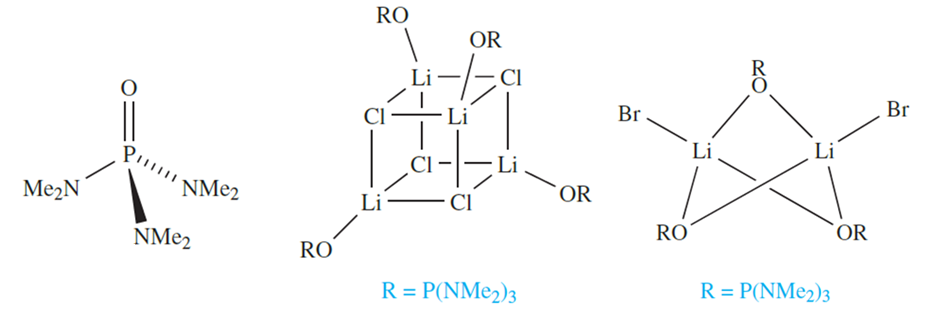
A growing number of complexes (generally air- and moisture- sensitive) involving alkali metal ions with O- or Ndonor ligands and formed in non-aqueous media are now known, although the chemistry of the later group 1 metals is not so widely developed as that of Li. A general method of synthesis is to prepare an alkali metal salt in the presence of a coordinating ligand. For example, in [{LiCl(HMPA)}4], use of the bulky ligand HMPA (hexamethylphosphoramide), 10.5, results in the isolation of a discrete complex rather than an extended LiCl lattice; the complex contains the cubic Li4Cl4 core. Increasing the size of the halogen tends to reduce the nuclearity of the product, e.g. [Li2Br2(HMPA)3]. The bonding in these complexes is of interest can be viewed in terms of a central aggregate of Li+ and Cl- ions, and in general, the bonding should be considered to be predominantly ionic.
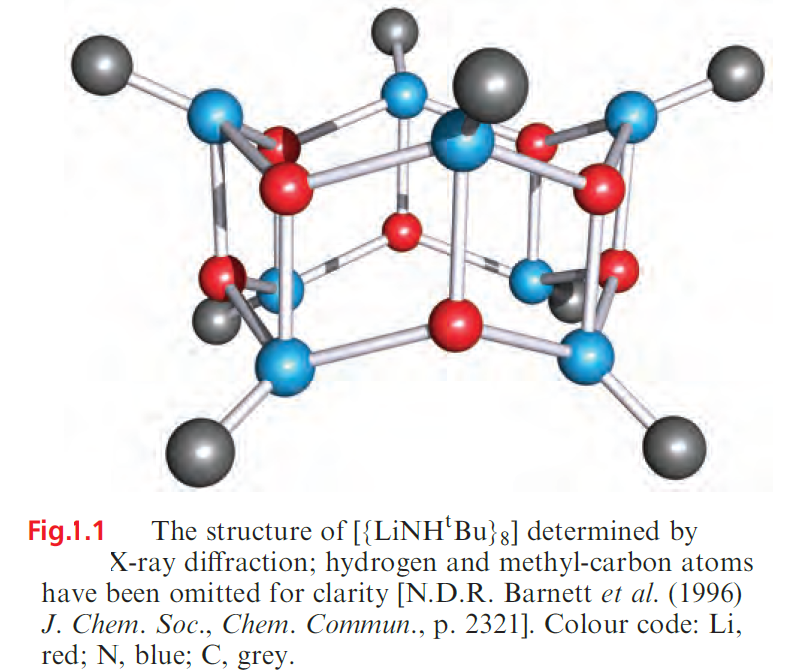
Amidolithium complexes of type RR’NLi (e.g. R and R’ = alkyl, aryl, silyl) exhibit a fascinating structural diversity; as above, bulky amido ligands are essential for complex stabilization. Planar Li2N2-rings are common structural units, and these appear in a variety of laddered structures which may be polymeric or discrete molecular as in [{tBuHNLi}8] (Figure 1.1).



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|