
Saline hydrides
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Inorganic Chemistry
المصدر:
Inorganic Chemistry
 الجزء والصفحة:
p 251
الجزء والصفحة:
p 251
 9-1-2018
9-1-2018
 2004
2004
Saline hydrides
Saline hydrides are formed when the group 1 or 2 metals (except Be) are heated with H2. All are white, high melting solids (e.g. LiH, mp = 953 K; NaH, mp = 1073K with decomposition); the group 1 hydrides crystallize with the NaCl lattice, and the presence of the H- ion is indicated by the good agreement between lattice energies obtained from Born–Haber cycles and from X-ray and compressibility data. Additional evidence comes from the fact that the electrolysis of molten LiH liberates H2 at the anode.
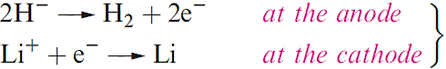
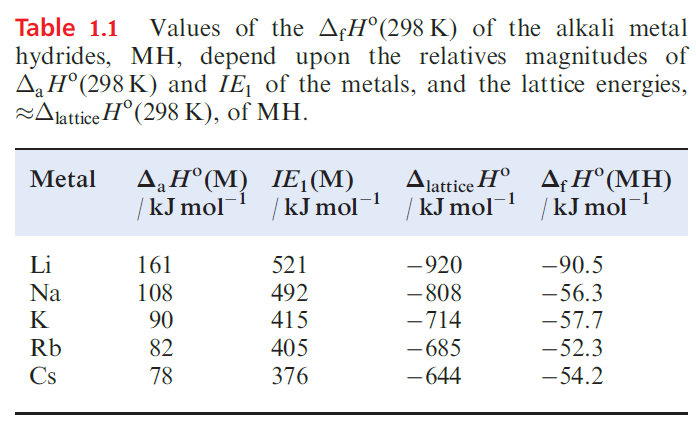
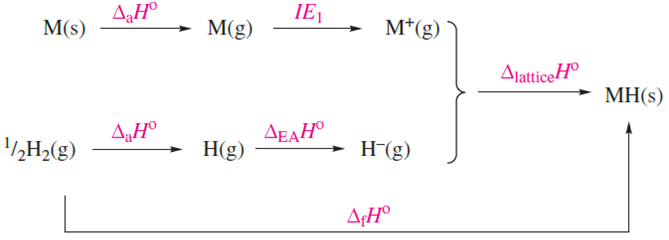
The reactivity of the group 1 hydrides increases with an increase in atomic number and ionic size of the metal; in keeping with this, values of ΔfHo become less negative, with that of LiH being significantly more negative than those of the other alkali metal hydrides. Table 9.5 lists factors that contribute towards this trend. Since the hydride ion is a common factor in the series, we need to look at the extent to which the value of ΔlatticeHo offsets the sum of ΔaHo and IE1 in order to reconcile the trend in values of ΔfHo .
The H- ion is similar in size to F -, and thus the trend parallels that observed for alkali metal fluorides.
Saline hydrides react immediately with protic solvents such as H2O, NH3 or EtOH, showing that the H- ion is an extremely strong base. Widespread use is made of NaH and KH as deprotonating agents.
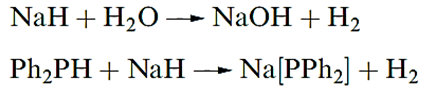
Of the saline hydrides, LiH, NaH and KH are the most commonly used, but their moisture sensitivity means that reaction conditions must be water-free. Of particular significance are the reactions between LiH and Al2Cl6 to give lithium tetrahydridoaluminate(1_), Li[AlH4], (also called lithium aluminium hydride or lithal ) and between NaH and B(OMe)3 or BCl3 to give sodium tetrahydroborate(1_), commonly known as sodium borohydride. The compounds Li[AlH4], Na[BH4] and NaH find wide applications as reducing agents.

 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


