
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-7-2017
Date: 22-8-2017
Date: 11-9-2017
|
NITRATION OF BENZENE (Nitrobenzene [C6H5NO2])
Similar to the alkylation and the chlorination of benzene, the nitration reaction is an electrophilic substitution of a benzene hydrogen (a proton) with a nitronium ion (NO2+). The liquid-phase reaction occurs in presence of both concentrated nitric and sulfuric acids at approximately 50°C. Concentrated sulfuric acid has two functions: it reacts with nitric acid to form the nitronium ion, and it absorbs the water formed during the reaction, which shifts the equilibrium to the formation of nitrobenzene:
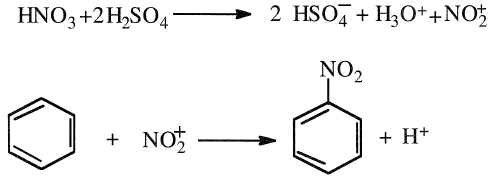
Most of the nitrobenzene (≈97%) produced is used to make aniline. Other uses include synthesis of quinoline, benzidine, and as a solvent for cellulose ethers.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|