

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Bond Angles
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 67
6-7-2017
2501
Bond Angles
Valence-shell electron pair repulsion (VSEPR) theory gives reasonably accurate predictions of bond angles in a molecule. VSEPR theory uses a simple electrostatic model in which groups of electrons around a central atom repel one another and occupy positions as far apart as possible. The number of
electron groups, called the steric number (SN), is the number of unshared electron pairs plus the number of bonding groups; a bonding group is defined as the 2 electrons of a single bond, the 4 electrons of a double bond, or the 6 electrons of a triple bond. Thus, for example, HC≡CH has 2 electrons in each C-H bond and 6 electrons in the C≡C bond, so SN(C) = 2. The two groups of electrons get as far away from each other as possible—on opposite sides of the C atom. VSEPR theory predicts a linear molecule. The other predictions of VSEPR theory are as follows:
• SN = 2, linear molecule, 180° bond angles,
• SN = 3, trigonal planar, 120° bond angles,
• SN = 4, tetrahedral, 109.5° bond angles,
• SN = 5, trigonal bipyramidal, 120 & 90° angles
• SN = 6, octahedral, 90 & 180° bond angles.
The basic molecular shapes are shown in Figure 1.1. Note that the words tetrahedral and octahedral refer to the four- and eight-sided solids formed by connecting the ligand atoms, not to the coordination numbers, which are 4 and 6, respectively.
Bond angles are sometimes rationalized by invoking atomic orbital hydbridization. Thus 180° angles are said to result from sp hybrids, 120° angles from sp2 hybrids, and 109.5° angles from sp3 hybrids. This concept of hybrid orbitals is fundamentally flawed, but it has become a part of the language of chemists. You will no doubt hear of hybrid orbitals; take sp, sp2, and sp3 as synonyms for 180°, 120°, 109.5°.
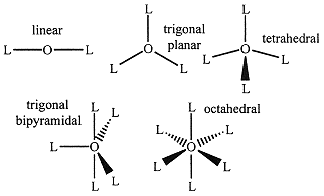
Figure 1.1. The Five VSEPR Molecular Shapes.
If all ligands are the same, the VSEPR bond angle predictions are almost always exact. Thus in CH4 (SN = 4) and SF6 (SN = 6), the structures are perfectly tetrahedral and octahedral, respectively. In NH3 and H2O, both with SN = 4, Ð H-N-H = 107.3°, Ð H-O-H = 104.5°. This departure from the predicted 109.5° angle is due to the greater repulsion of the lone pairs on N and O. Similarly, the two electron pairs in a double bond are more repulsive than a single bonding pair so that the H-C-H bond angle in H2C=CH2 is 116.6°, slightly smaller than the 120° angle predicted for SN = 3.
For linear, trigonal planar, tetrahedral, and octahedral structures, all ligand positions are equivalent. The trigonal bipyramidal structure, on the other hand, has two kinds of ligand sites—axial and equatorial.
Each axial electron pair is 90° away from three equatorial pairs and 180° away from the other axial pair. Each equatorial pair is 90° away from two axial pairs and 120° from the other two equatorial pairs. Electron repulsion is slightly greater for the axial pairs, so that a more repulsive electron pair is better off at an equatorial site. Thus for an SN = 5 molecule with an unshared electron pair such as SF4, the unshared pair will occupy an equatorial site. Highly electronegative ligand atoms such as F form polar bonds with the bonding pair further from the central atom; these bonding pairs are thus less repulsive than bonding pairs associated with less polar bonds. Thus in PF2Cl3, the F atoms will occupy axial positions.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















