


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-6-2020
Date: 6-7-2017
Date: 27-4-2019
|
Bond Lengths
The distance between two atoms of the same type is approximately constant from one compound to another if the bond order is the same. The length of a single covalent bond can be estimated by the sum of the covalent radii of the two atoms. A short list of covalent radii is given in Table 1.1.
The lengths of the C-C bonds in H3C-CH3, H2C=CH2, and HC≡CH are 154, 133, and 120 pm, respectively. For atoms similar in size to C, a double bond is about 21 pm shorter and a triple bond is 34
Table 1.1. Single-Bond Covalent Radii (pm)
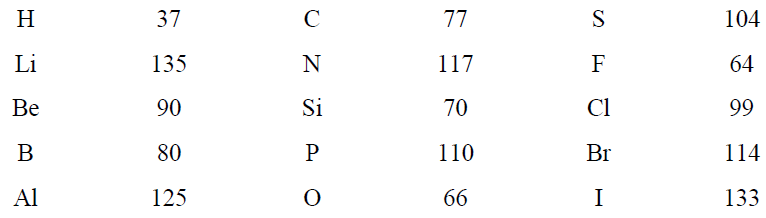
Table 1.2. Examples of Bond Length Estimation
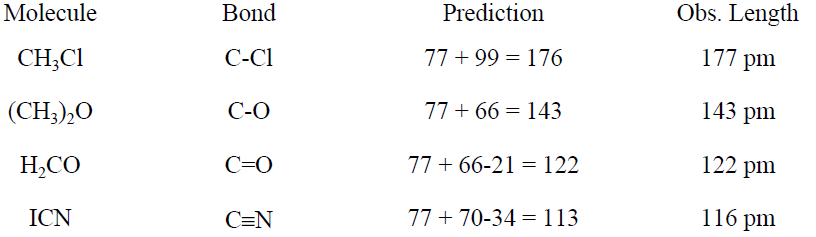
pm shorter than a single bond between the same atoms. In the case of resonance, a bond length is approximately the average of the values expected for the resonance structures. Some applications of this rule are shown in Table 1.2.
You Need to Know
Length of single bond = sum of covalent radii, double bonds about 21 pm shorter, triple bonds about 34 pm shorter. Average lengths for resonance structures to get approximate bond length for molecule.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|