

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
SN2 reactions- The Leaving Group
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th. p 319
29-5-2017
3409
SN2 reactions- The Leaving Group
Still another variable that can affect the SN2 reaction is the nature of the group displaced by the incoming nucleophile. Because the leaving group is expelled with a negative charge in most SN2 reactions, the best leaving groups are those that best stabilize the negative charge in the transition state. The greater the extent of charge stabilization by the leaving group, the lower the energy of the transition state and the more rapid the reaction. But the groups that best stabilize a negative charge are also the weakest bases. Thus, weak bases such as Cl-, Br-, and tosylate ion make good leaving groups, while strong bases such as OH2 and NH2- make poor leaving groups.
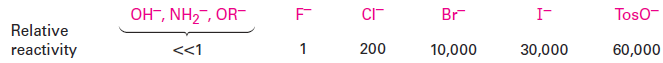
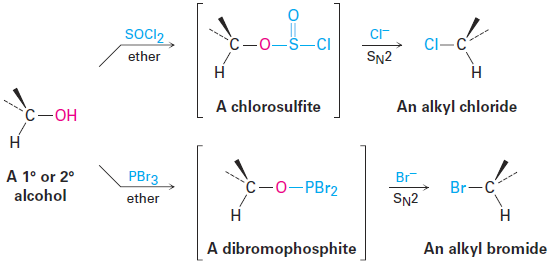
It’s just as important to know which are poor leaving groups as to know which are good, and the preceding data clearly indicate that F-, HO-, RO-, and H2N- are not displaced by nucleophiles. In other words, alkyl fluorides, alcohols, ethers, and amines do not typically undergo SN2 reactions. To carry out an SN2 reaction with an alcohol, it’s necessary to convert the -OH into a better leaving group. This, in fact, is just what happens when a primary or secondary alcohol is converted into either an alkyl chloride by reaction with SOCl2 or an alkyl bromide by reaction with PBr3.
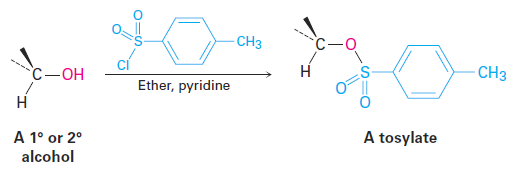
Alternatively, an alcohol can be made more reactive toward nucleophilic substitution by treating it with para-toluenesulfonyl chloride to form a tosylate.
As noted previously, tosylates are even more reactive than halides in nucleophilic substitutions. Note that tosylate formation does not change the configuration of the oxygen-bearing carbon because the C - O bond is not broken.
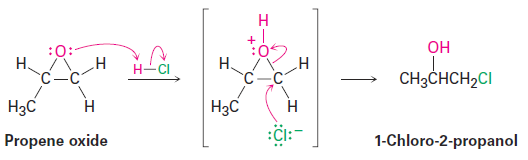
The one general exception to the rule that ethers don’t typically undergo SN2 reactions pertains to epoxides, the three-membered cyclic ethers. Because of the angle strain in their three-membered ring, epoxides are much more reactive than other ethers. They react with aqueous acid to give 1,2-diols, and they react readily with many other nucleophiles as well. Propene oxide, for instance, reacts with HCl to give 1-chloro-2-propanol by a SN2 backside attack on the less hindered primary carbon atom.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















