

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Preparing Alkyl Halides from Alkanes: Radical Halogenation
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th. p 290
25-5-2017
1773
Preparing Alkyl Halides from Alkanes: Radical Halogenation
Simple alkyl halides can sometimes be prepared by reaction of an alkane with Cl2 or Br2 in the presence of light through a radical chain-reaction pathway. The mechanism is shown in Figure 1.1 for chlorination.

Figure 1.1 Mechanism of the radical chlorination of methane. Three kinds of steps are required: initiation, propagation, and termination. The propagation steps are a repeating cycle, with Cl· a reactant in step 1 and a product in step 2, and with ·CH3 aproduct in step 1 and a reactant in step 2. (The symbol hn shown in the initiation step is the standard way of indicating irradiation with light.)
The radical substitution reactions require three kinds of steps: initiation, propagation, and termination. Once an initiation step has started the process by producing radicals, the reaction continues in a self-sustaining cycle. The cycle requires two repeating propagation steps in which a radical, the halogen, and the alkane yield alkyl halide product plus more radical to carry on the chain. The chain is occasionally terminated by the combination of two radicals.
Although interesting from a mechanistic point of view, alkane halogenation is a poor synthetic method for preparing alkyl halides because mixtures of products invariably result. For example, chlorination of methane does not stop cleanly at the monochlorinated stage but continues to give a mixture of dichloro, trichloro, and even tetrachloro products.

The situation is even worse for chlorination of alkanes that have more than one kind of hydrogen. For example, chlorination of butane gives two monochlorinated products in a 30;70 ratio in addition to dichlorobutane, trichlorobutane, and so on.

As another example, 2-methylpropane yields 2-chloro-2-methylpropane and 1-chloro-2-methylpropane in a 35;65 ratio, along with more highly chlorinated products.

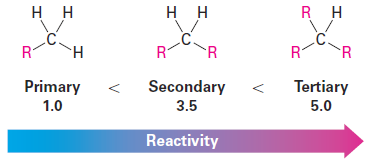
From these and similar reactions, it’s possible to calculate a reactivity order toward chlorination for different kinds of hydrogen atoms in a molecule.
Take the butane chlorination, for instance. Butane has six equivalent primary hydrogens ( - CH3) and four equivalent secondary hydrogens ( - CH2 - ). The fact that butane yields 30% of 1-chlorobutane product means that each one of the six primary hydrogens is responsible for 30% / 6 = 5% of the product. Similarly, the fact that 70% of 2-chlorobutane is formed means that each of the four secondary hydrogens is responsible for 70% / 4 = 17.5% of the product. Thus, a secondary hydrogen reacts 17.5% / 5% = 3.5 times as often as a primary hydrogen.
A similar calculation for the chlorination of 2-methylpropane indicates that each of the nine primary hydrogens accounts for 65% / 9 = 7.2% of the product, while the single tertiary hydrogen (R3CH) accounts for 35% of the product. Thus, a tertiary hydrogen is 35% / 7.2% = 5 times as reactive as a primary hydrogen toward chlorination.
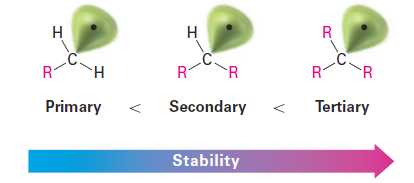
The data show that a tertiary C - H bond (400 kJ/mol; 96 kcal/mol) is weaker than a secondary C - H bond (410 kJ/mol; 98 kcal/mol), which is in turn weaker than a primary C - H bond (421 kJ/mol; 101 kcal/mol). Since less energy is needed to break a tertiary C - H bond than to break a primary or secondary C - H bond, the resultant tertiary radical is more stable than a primary or secondary radical.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















