
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Qualitative Description
المؤلف:
Donald A. Neamen
المصدر:
Semiconductor Physics and Devices
الجزء والصفحة:
p 115
18-5-2017
2035
Qualitative Description
We discussed the covalent bonding of silicon and considered the simple two-dimensional representation of the single-crystal silicon lattice as shown in Figure 1.1. Now consider adding a group V element, such as phosphorus, as a substitutional impurity. The group V element has five valence electrons. Four of these will contribute to the covalent bonding with the silicon atoms, leaving the fifth more loosely hound to the phosphorus atom. This effect is schematically shown in Figure 1.2. We refer to the fifth valence electron as a donor electron.
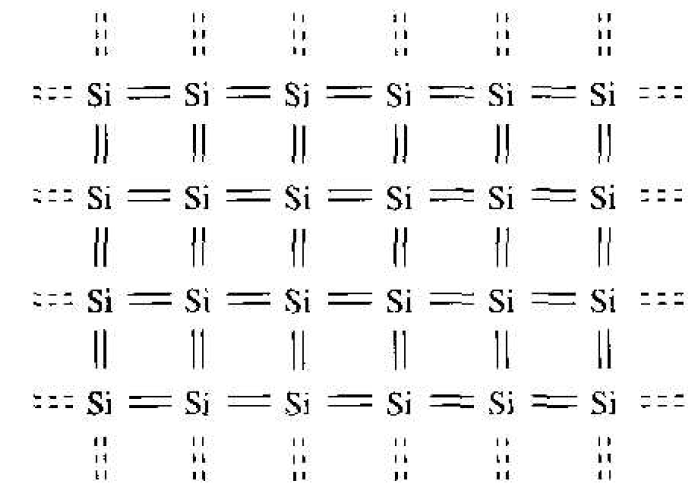
Figure 1.1 Two-dimensional representation of the intrinsic silicon lattice.

Figure 1.2 Two-dimensional representation of the silicon lattice doped with a phosphorus atom.
The phosphorus atom without the donor electron is positively charged. At very low temperatures, the donor electron is bound to the phosphorus atom. However. By intuition, it should seem clear that the energy required to elevate the donor electron into the conduction band is considerably less than that for the electrons involved in the covalent bonding. Figure 1.3 shows the energy-hand diagram that we would expect. The energy level, Ed, is the energy state of the donor electron.
If a small amount of energy, such as thermal energy. is added to the donor electron, it can be elevated into the conduction band, leaving behind a positively charged phosphorus ion. The electron in the conduction band can now move through the crystal generating a current, while the positively charged ion is fixed in the crystal. This type of impurity atom donates an electron to the conduction band and so is called a donor impurity atoms. The donor impurity atoms add electrons to the conduction band without creating holes in the valence band. The resulting material is referred to as an n-type semiconductor (n for the negatively charged electron).
Now consider adding a group III element, such as boron, as a substitutional impurity to silicon. The group III element has three valence electrons, which are all taken up in the covalent bonding. As shown in Figure 1.4a, one covalent bonding position appears to be empty. If an electron were to occupy this "empty" position. Its
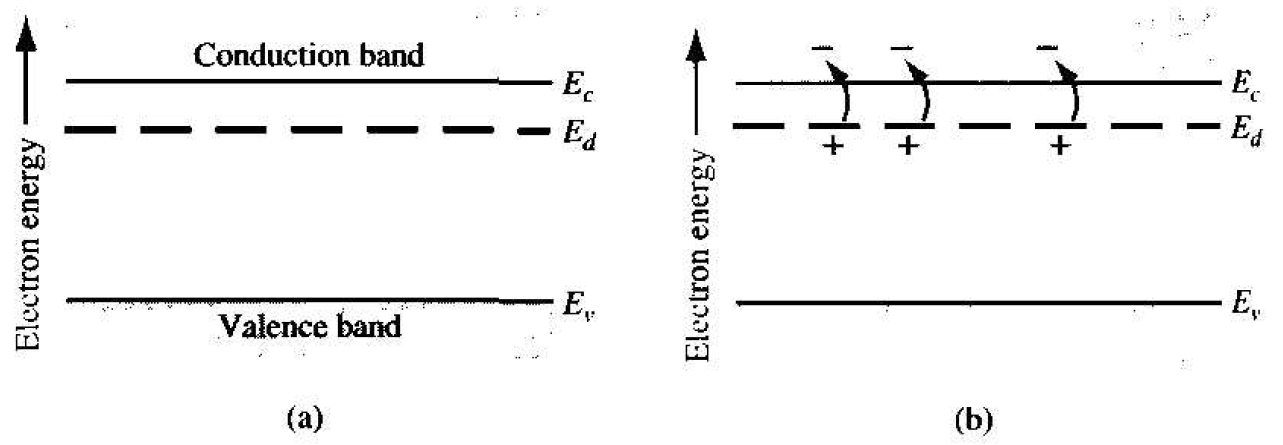
Figure 1.3 The energy-hand diagram showing (a) the discrete donor energy state and (b) the effect of a donor state being ionized.
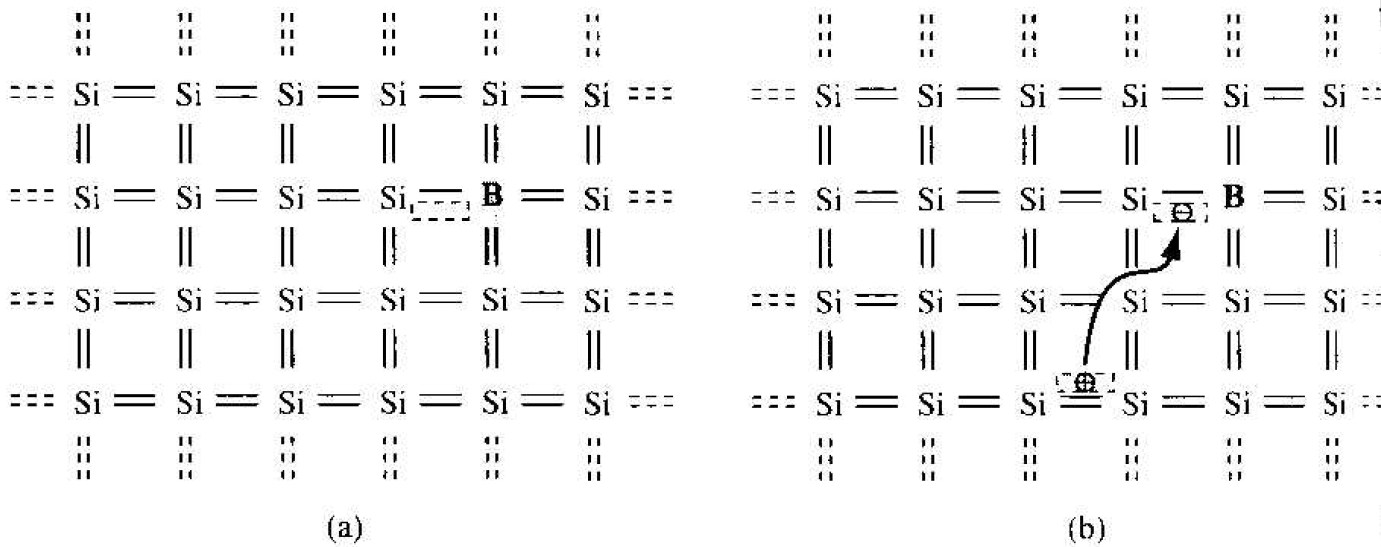
Figure 1.4 Two-dimensional representation of a silicon lattice (a) doped with a boron atm and (b) showing [he ionization of the boron atom resulting in a hole.

Figure 1.5 The energy-band diagram showing (a) the discrete acceptor energy state and (b) the effect of an acceptor state being ionized.
energy would have to be greater than that of the valence electrons, since the net charge state of the boron atom would now be negative. However, the electron occupying this "empty" position does not have sufficient energy to be in the conduction band, so its energy is far smaller than the conduction-band energy. Figure 1.4b shows how valence electrons may gain a small amount of thermal energy and move about in the crystal. The "empty" position associated with the boron atom becomes occupied, and other valence electron positions become vacated. These other vacated electron positions can he thought of as holes in the semiconductor material.
Figure 1.5 shows the expected energy state of the "empty" position and also the formation of a hole in the valence hand. The hole can move through the crystal generating a current, while the negatively charged boron atom is fixed in the crystal. The group Ill atom accepts an electron from the valence band and so is referred to as an acceptor impurity atom. The acceptor atom can generate holes in the valence hand without generating electrons in the conduction band. This type of semiconductor material is referred to as a p-type material (p for the positively charged hole).
The pure single-crystal semiconductor material is called an intrinsic material. Adding controlled amounts of dopant atoms, either donors or acceptors, creates a material called an extrinsic semiconductor. An extrinsic semiconductor will have either a preponderance of electrons (n type) or a preponderance of holes (p type).
 الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















