

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
How does the Hammond postulate apply to electrophilic addition reactions
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th. p 213
18-5-2017
2895
How does the Hammond postulate apply to electrophilic addition reactions
The structure of a transition state resembles the structure of the nearest stable species. Transition states for endergonic steps structurally resemble products, and transition states for exergonic steps structurally resemble reactants.
How does the Hammond postulate apply to electrophilic addition reactions? The formation of a carbocation by protonation of an alkene is an endergonic step. Thus, the transition state for alkene protonation structurally resembles the carbocation intermediate, and any factor that stabilizes the carbocation will also stabilize the nearby transition state. Since increasing alkyl substitution stabilizes carbocations, it also stabilizes the transition states leading to those ions, thus resulting in a faster reaction. In other words, more stable carbocations form faster because their greater stability is reflected in the lower-energy transition state leading to them (Figure 1.1).
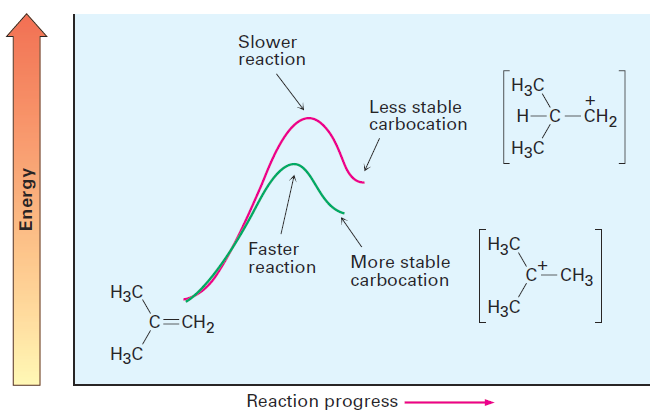
Figure 1.1 Energy diagrams for carbocation formation. The more stable tertiary carbocation is formed faster (green curve) because its increased stability lowers the energy of the transition state leading to it.
We can imagine the transition state for alkene protonation to be a structure in which one of the alkene carbon atoms has almost completely rehybridized from sp2 to sp3 and the remaining alkene carbon bears much of the positive charge (Figure 1.2). This transition state is stabilized by hyperconjugation and inductive effects in the same way as the product carbocation. The more alkyl groups that are present, the greater the extent of stabilization and the faster the transition state forms.
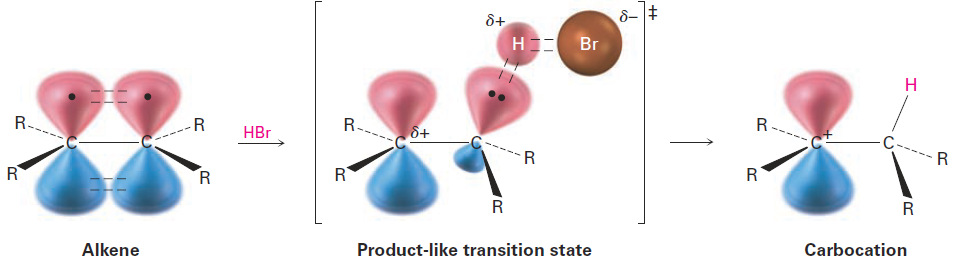
Figure 1.2 The hypothetical structure of a transition state for alkene protonation. The transition state is closer in both energy and structure to the carbocation than to the alkene.
Thus, an increase in carbocation stability (lower ΔG°) also causes an increase in transition-state stability (lower ΔG‡), thereby increasing the rate of its formation.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















