
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-5-2017
Date: 4-5-2017
Date: 16-4-2017
|
Parts per Million and Parts per Billion
For very dilute solutions, parts per million (ppm) is a convenient way to express concentration:
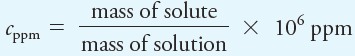
where cppm is the concentration in parts per million. The units of mass in the numerator and denominator must agree so that they cancel. For even more dilute solutions, 109 ppb rather than 106 ppm is used in the previous equation to give the results in parts per billion (ppb). The term parts per thousand (ppt) is also used, especially in oceanography.
Example :What is the molar concentration of K+ in a solution that contains 63.3 ppm of K3Fe(CN)6 (329.3 g/mol)?
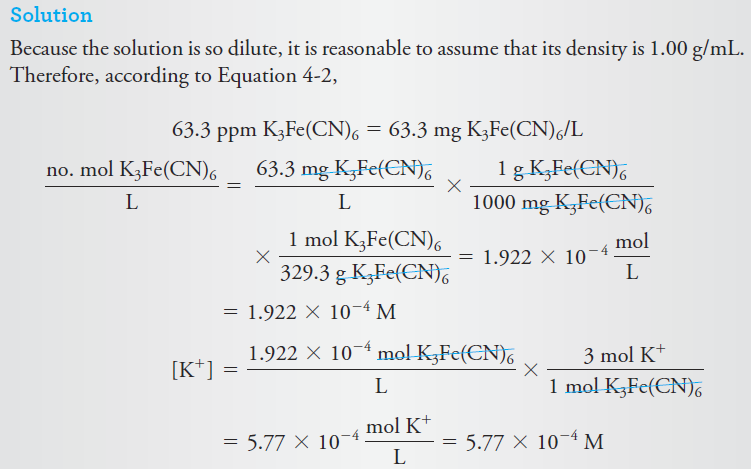



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|