
Percent Concentration
 المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
 المصدر:
Fundamentals of Analytical Chemistry
المصدر:
Fundamentals of Analytical Chemistry
 الجزء والصفحة:
9th. p 70
الجزء والصفحة:
9th. p 70
 19-4-2017
19-4-2017
 4868
4868
Percent Concentration
Chemists frequently express concentrations in terms of percent (parts per hundred). Unfortunately, this practice can be a source of ambiguity because percent composition of a solution can be expressed in several ways. Three common methods are
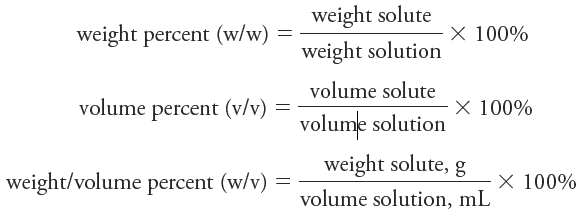
Note that the denominator in each of these expressions is the mass or volume of solution rather than mass or volume of solvent. Note also that the first two expressions do not depend on the units used for weight (mass) as long as the same units are used in the numerator and the denominator. In the third expression, units must be defined because the numerator and denominator have different units that do not cancel. Of the three expressions, only weight percent has the advantage of being temperature independent.
Weight percent is often used to express the concentration of commercial aqueous reagents. For example, nitric acid is sold as a 70% (w/w) solution, meaning that the reagent contains 70 g of HNO3 per 100 g of. Volume percent is commonly used to specify the concentration of a solution prepared by diluting a pure liquid compound with another liquid. For example, a 5% (v/v) aqueous solution of methanol usually describes a solution prepared by diluting 5.0 mL of pure methanol with enough water to give 100 mL.
Weight or volume percent is often used to indicate the composition of dilute aqueous solutions of solid reagents. For example, 5% (w/v) aqueous silver nitrate often refers to a solution prepared by dissolving 5 g of silver nitrate in sufficient water to give 100 mL of solution.
To avoid uncertainty, always specify explicitly the type of percent composition being discussed. If this information is missing, the investigator must decide intuitively which of the several types is to be used. The potential error resulting from a wrong choice is considerable. For example, commercial 50% (w/w) sodium hydroxide contains 763 g NaOH per liter, which corresponds to 76.3% (w/v) sodium hydroxide.
 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


