

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydrogen Bonding Gives Water Its Unusual Properties
المؤلف:
David L. Nelson, Michael M. Cox
المصدر:
Book or Source : Lehninger Principles of Biochemistry 6th ed 2012
الجزء والصفحة:
p 47
12-4-2017
9848
Hydrogen Bonding Gives Water Its Unusual Properties
Water has a higher melting point, boiling point, and heat of vaporization than most other common solvents (Table 1.1). These unusual properties are a consequence of attractions between adjacent water molecules that give liquid water great internal cohesion.
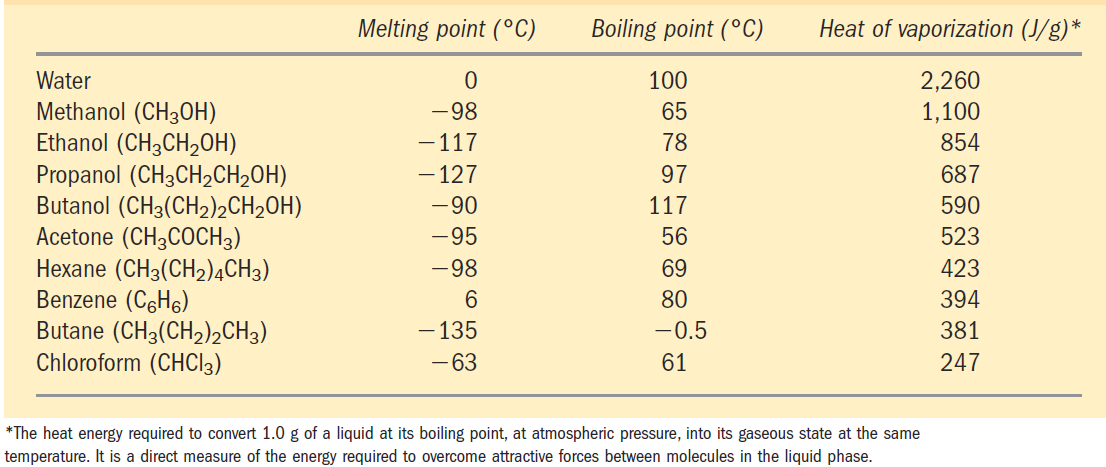
TABLE 1.1 Melting Point, Boiling Point, and Heat of Vaporization of Some Common Solvents
A look at the electron structure of the H2O molecule reveals the cause of these intermolecular attractions. Each hydrogen atom of a water molecule shares an electron pair with the central oxygen atom. The geometry of the molecule is dictated by the shapes of the outer electron orbitals of the oxygen atom, which are similar to the sp3 bonding orbitals of carbon. These orbitals describe a rough tetrahedron, with a hydrogen atom at each of two corners and unshared electron pairs at the other two corners (Fig. 1.1a). The HOOH bond angle is 104.5ο, slightly less than the 109.5ο of a perfect tetrahedron because of crowding by the nonbonding orbitals of the oxygen atom. The oxygen nucleus attracts electrons more strongly than does the hydrogen nucleus (a proton); that is, oxygen is more electronegative. The sharing of electrons between H and O is therefore unequal; the electrons are more often in the vicinity of the oxygen atom than of the hydrogen. The result of this unequal electron sharing is two electric dipoles in the water molecule, one along each of the HOO bonds; each hydrogen bears a partial positive charge (δ+) and the oxygen atom bears a partial negative charge equal to the sum of the two partial positives (2δ-). As a result, there is an electrostatic attraction between the oxygen atom of one water molecule and the hydrogen of another (Fig. 1.1c), called a hydrogen bond. Throughout this book, we represent hydrogen bonds with three parallel blue lines, as in Figure 1.1c. Hydrogen bonds are relatively weak. Those in liquid water have a bond dissociation energy (the energy required to break a bond) of about 23 kJ/mol, compared with 470 kJ/mol for the covalent OOH bond in
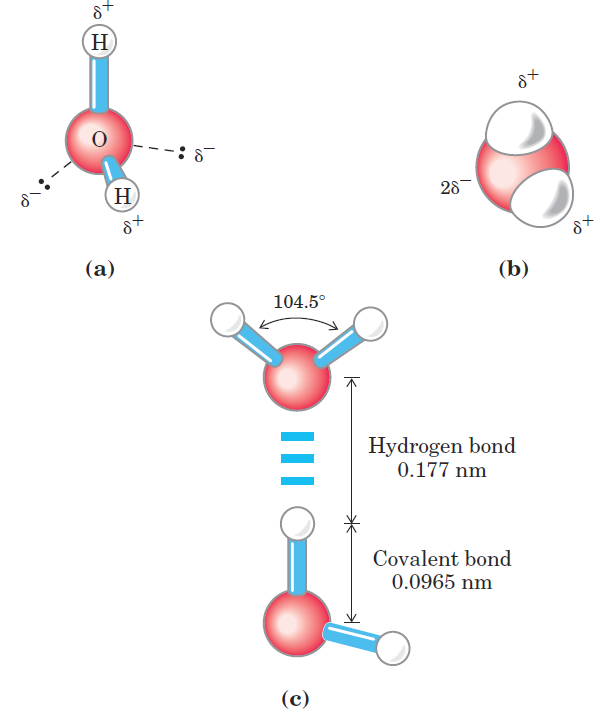
FIGURE 1.1 Structure of the water molecule. The dipolar nature of the H2O molecule is shown by (a) ball-and-stick and (b) space-filling models. The dashed lines in (a) represent the nonbonding orbitals. There is a nearly tetrahedral arrangement of the outer-shell electron pairs around the oxygen atom; the two hydrogen atoms have localized partial positive charges (δ+) and the oxygen atom has a partial negative charge (2δ-). (c) Two H2O molecules joined by a hydrogen bond (designated here, and throughout this book, by three blue lines) between the oxygen atom of the upper molecule and a hydrogen atom of the lower one. Hydrogen bonds are longer and weaker than covalent OOH bonds.
water or 348 kJ/mol for a covalent COC bond. The hydrogen bond is about 10% covalent, due to overlaps in the bonding orbitals, and about 90% electrostatic. At room temperature, the thermal energy of an aqueous solution (the kinetic energy of motion of the individual atoms and molecules) is of the same order of magnitude as that required to break hydrogen bonds. When water is heated, the increase in temperature reflects the faster motion of individual water molecules. At any given time, most of the molecules in liquid water are engaged in hydrogen bonding, but the lifetime of each hydrogen bond is just 1 to 20 picoseconds (1 ps =10-12 s); upon breakage of one hydrogen bond, another hydrogen bond forms, with the same partner or a new one, within 0.1 ps. The apt phrase “flickering clusters” has been applied to the short-lived groups of water molecules interlinked by hydrogen bonds in liquid water. The sum of all the hydrogen bonds between H2O molecules confers great internal cohesion on liquid water. Extended networks of hydrogen-bonded water molecules also form bridges between solutes (proteins and nucleic acids, for example) that allow the larger molecules to interact with each other over distances of several nanometers without physically touching. The nearly tetrahedral arrangement of the orbitals about the oxygen atom (Fig. 1.1a) allows each water molecule to form hydrogen bonds with as many as four neighboring water molecules. In liquid water at room temperature and atmospheric pressure, however, water molecules are disorganized and in continuous motion, so that each molecule forms hydrogen bonds with an average of only 3.4 other molecules. In ice, on the other hand, each water molecule is fixed in space and forms hydrogen bonds with a full complement of four other water molecules to yield a regular lattice structure (Fig. 1.2). Breaking a sufficient proportion of hydrogen bonds to destabilize the crystal lattice of ice requires much thermal energy, which accounts for the relatively high melting point of water (Table 1.1). When ice melts or water evaporates, heat is taken up by the system:
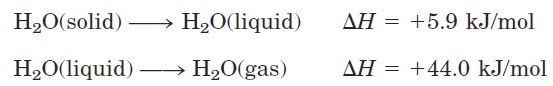
During melting or evaporation, the entropy of the aqueous system increases as more highly ordered arrays of water molecules relax into the less orderly hydrogen bonded arrays in liquid water or the wholly disordered gaseous state. At room temperature, both the melting of ice and the evaporation of water occur spontaneously; the tendency of the water molecules to associate through hydrogen bonds is outweighed by the energetic push toward randomness. Recall that the free-energy change (ΔG) must have a negative value for a process to occur spontaneously:ΔG = ΔH -TΔS, where ΔG represents the driving force, ΔH the enthalpy change from making and breaking bonds, and ΔS the change in randomness and breaking bonds, and ΔS the change in randomness. Because ΔH is positive for melting and evaporation, it is clearly the increase in entropy (ΔS) that makes ΔG negative and drives these transformations.
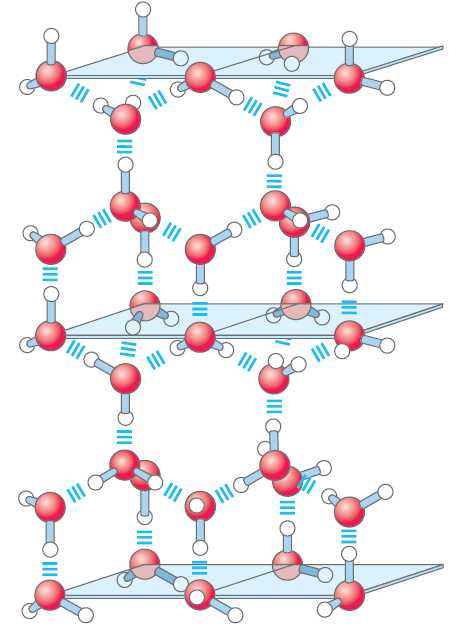
FIGURE 1.2 Hydrogen bonding in ice. In ice, each water molecule forms the maximum of four hydrogen bonds, creating a regular crystal lattice. By contrast, in liquid water at room temperature and atmospheric pressure, each water molecule hydrogen-bonds with an average of 3.4 other water molecules. This crystal lattice of ice makes it less dense than liquid water, and thus ice floats on liquid water.
 الاكثر قراءة في مواضيع عامة في الكيمياء الحياتية
الاكثر قراءة في مواضيع عامة في الكيمياء الحياتية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















