


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-2-2017
Date: 2-2-2018
Date: 9-2-2018
|
chemical properties of silicon, germanium, tin and lead Structures
Chemical properties Silicon is much more reactive than carbon. At high temperatures, Si combines with O2, F2, Cl2, Br2, I2, S8, N2, P4, C and B to give binary compounds. Silicon liberates H2 from aqueous alkali (equation 1.1), but is insoluble in acids other than a mixture of concentrated HNO3 and HF.

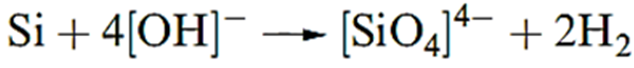 (1.1)
(1.1)
On descending group 14, the electropositivity and reactivity of the elements increase. In general, Ge behaves in a similar manner to Si, but, being more electropositive, reacts with concentrated HNO3 (forming GeO2), and does not react with aqueous alkali. Reactions between Ge and HCl or H2S yield GeCl4 or GeS2 respectively. Although high temperatures are needed for reactions between Sn and O2 (to give SnO2) or sulfur (giving SnS2), the metal reacts readily with halogens to yield SnX4. Tin is little affected by dilute HCl or H2SO4, but reacts with dilute HNO3 (to give Sn(NO3)2 and NH4NO3) and with concentrated acids yielding SnCl2 (from HCl) and SnSO4 and SO2 (from H2SO4). Hot aqueous alkali oxidizes the metal to Sn(IV) according to equation 1.2.

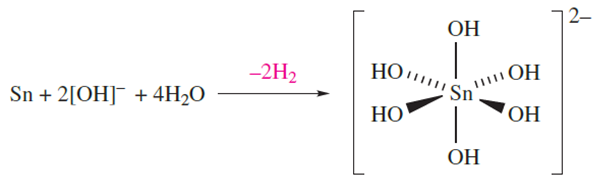 (1.2)
(1.2)
A pyrophoric material is spontaneously inflammable. When finely divided, Pb is pyrophoric, but bulk pieces are passivated by coatings of, for example, PbO, and reaction with O2 in air occurs only above ≈ 900 K. Lead reacts very slowly with dilute mineral acids, slowly evolves H2 from hot concentrated HCl, and reacts with concentrated HNO3 to give Pb(NO3)2 and oxides of nitrogen. For reactions of Pb with halogens.
Worked example : Reactivity of the group 14 elements with halogens
Write an equation for the reaction that takes place when Si is heated in F2. The product of the reaction is a gas for which ΔfHo(298 K) = _1615 kJ mol-1. Use this value and appropriate data from the Appendices in the book to calculate a value for the Si–F bond enthalpy.
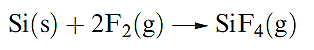
To find the bond enthalpy term, start by writing an equation for the dissociation of gaseous SiF4 into gaseous atoms, and then set up an appropriate thermochemical cycle that incorporates ΔfHo (SiF4,g).

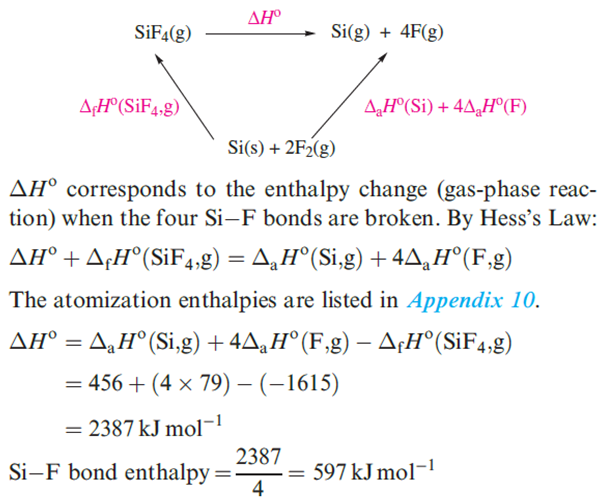



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
علي بابا تطلق نماذج "Qwen" الجديدة في أحدث اختراق صيني لمجال الذكاء الاصطناعي مفتوح المصدر
|
|
|
|
|
|
|
ضمن برنامج تأهيل المنتسبين الجدد قسم الشؤون الدينية يقدم محاضرات فقهية وعقائدية لنحو 130 منتسبًا
|
|
|