
Thiosulfuric acid, H2S2O3, and polythionates
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 462
الجزء والصفحة:
2th ed p 462
 22-3-2017
22-3-2017
 2807
2807
Thiosulfuric acid, H2S2O3, and polythionates
Thiosulfuric acid may be prepared under anhydrous conditions by reaction 1.1, or by treatment of lead thiosulfate (PbS2O3) with H2S, or sodium thiosulfate with HCl. The free acid is very unstable, decomposing at 243K or upon contact with water.
 (1.1)
(1.1)
Arepresentation of the structure of thiosulfuric acid is given in Table 15.8, but the conditions of reaction 1.1 may suggest protonation at sulfur, i.e. (HO)(HS)SO2. Thiosulfate salts are far more important than the acid; crystallization of the aqueous solution from reaction 1.2 yields Na2S2O3.5H2O.
 (1.2)
(1.2)

(1.1)
The thiosulfate ion, 1.1, is a very good complexing agent for Ag, and Na2S2O3 is used in photography for removing unchanged AgBr from exposed photographic film (equation 1.3 ). In the complex ion [Ag(S2O3)3]5- , each thiosulfate ion coordinates to Ag through a sulfur donor atom.
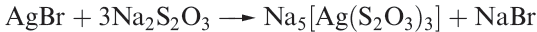 (1.3)
(1.3)
Most oxidizing agents (including Cl2 and Br2) slowly oxidize [S2O3]2- to [SO4] 2-, and Na2S2O3 is used to remove excess Cl2 in bleaching processes. In contrast, I2 rapidly oxidizes [S2O3] 2- to tetrathionate; reaction 1.4 is of great importance in titrimetric analysis.
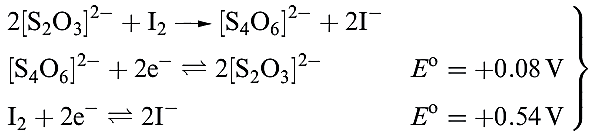 (1.4)
(1.4)
Polythionates contain ions of type [SnO6]2- and may be prepared by condensation reactions such as those in scheme 1.5, but some ions must be made by specific routes. Polythionate ions are structurally similar and have two {SO3}- groups connected by a sulfur chain (1.2 shows [S5O6]2-); solid state structures for a number of salts show chain conformations are variable. In aqueous solution, polythionates slowly decompose to H2SO4, SO2 and sulfur.
 (1.5)
(1.5)
Some compounds are known in which S atoms in a polythionate are replaced by Se or Te, e.g. Ba[Se(SSO3)2] and Ba[Te(SSO3)2] . Significantly, Se and Te cannot replace the terminal S atoms, presumably because in their highest oxidation states, they are too powerfully oxidizing and attack the remainder of the chain.
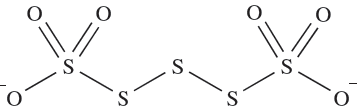
(1.2)
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


