
Oxides of sulfur
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 444
الجزء والصفحة:
2th ed p 444
 20-3-2017
20-3-2017
 1908
1908
Oxides of sulfur
The most important oxides of sulfur are SO2 and SO3, but there are also a number of unstable oxides. Among these are S2O (1.1) and S8O (1.2), made by reactions 1.1 and 1.2; the oxides SnO (n = 6–10) can be prepared by reaction 1.3, exemplified for S8O.

(1.1) (1.2)
 (1.1)
(1.1)
 (1.2)
(1.2)
 (1.3)
(1.3)
Sulfur dioxide is manufactured on a large scale by burning sulfur (the most important process) or H2S, by roasting sulfide ores (e.g. equation 1.4), or reducing CaSO4 (equation 1.5). Desulfurization processes to limit SO2 emissions and reduce acid rain (see Box 15.5) are now in use. In the laboratory, SO2 may be prepared by, for example, reaction 1.6, and it is commercially available in cylinders.
 (1.4)
(1.4)
 (1.5)
(1.5)
 (1.6)
(1.6)
Selected physical properties of SO2 are listed in Table 1.1.
Table 1.1 Selected physical properties of SO2 and SO3.

At 298 K, SO2 is a liquid and a good solvent (see Section 8.5). Sulfur dioxide has a molecular structure (1.3).
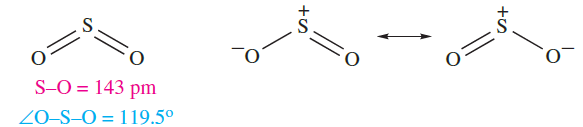
(1.3)
Sulfur dioxide reacts with O2 (see below), F2 and Cl2 (equation 1.7). It also reacts with the heavier alkali metal fluorides to give metal fluorosulfites (equation 1.8), and with CsN3 to give the Cs salt of [SO2N3]- (Figure 1.1).

Fig. 1.1 The structure of the azidosulfite anion, [SO2N3]-, determined by X-ray diffraction at 173K for the Cs salt [K.O. Christe et al. (2002) Inorg. Chem., vol. 41, p. 4275]. Colour code: N, blue; S, yellow; O, red.
 (1.7)
(1.7)
 (1.8)
(1.8)
In aqueous solution, it is converted to only a small extent to sulfurous acid; aqueous solutions of H2SO3 contain significant amounts of dissolved SO2 (see equations 6.18–6.20). Sulfur dioxide is a weak reducing agent in acidic solution, and a slightly stronger one in basic media (equations 1.9 and 1.10).
 (1.9)
(1.9)
 (1.10)
(1.10)
Thus, aqueous solutions of SO2 are oxidized to sulfate by many oxidizing agents (e.g. I2, [MnO4]-, [Cr2O7]2- and Fe3+ in acidic solutions). However, if the concentration of H is very high, [SO4]2- can be reduced to SO2 as in, for example, reaction 1.11; the dependence of E on [H+] was detailed in Section 7.2.
 (1.11)
(1.11)
In the presence of concentrated HCl, SO2 will itself act as an oxidizing agent; in reaction 1.12, the Fe(III) produced is then complexed by Cl-.
 (1.12)
(1.12)
The oxidation of SO2 by atmospheric O2 (equation 1.13) is very slow, but is catalysed by V2O5. This is the first step in the Contact process for the manufacture of sulfuric acid; operating conditions are crucial since equilibrium 1.13 shifts further towards the left-hand side as the temperature is raised, although the yield can be increased somewhat by use of high pressures of air. In practice, the industrial catalytic process operates at ≈750K and achieves conversion factors >98%.
 (1.13)
(1.13)
In the manufacture of sulfuric acid, gaseous SO3 is removed from the reaction mixture by passage through concentrated H2SO4, in which it dissolves to form oleum (see Section 15.9). Absorption into water to yield H2SO4 directly is not a viable option; SO3 reacts vigorously and very exothermically with H2O, forming a thick mist. On a small scale, SO3 can be prepared by heating oleum.

(1.4) (1.5)
Table 1.1 lists selected physical properties of SO3. In the gas phase, it is an equilibrium mixture of monomer (planar molecules, 1.4) and trimer. Resonance structures 1.5 are consistent with three equivalent S_O bonds, and with the S atom possessing an octet of electrons. Solid SO3 is polymorphic, with all forms containing SO4-tetrahedra sharing two oxygen atoms. Condensation of the vapour at low temperatures yields γ-SO3 which contains trimers (Figure 1.2a); crystals of γ-SO3 have an ice-like appearance. In the presence of traces of water, white crystals of β-SO3 form; β -SO3 consists of polymeric chains (Figure 1.2b), as does α-SO3 in which the chains are arranged into layers in the solid state lattice. Differences in the thermodynamic properties of the different polymorphs are very small, although they do react with water at different rates. Sulfur trioxide is very reactive and representative reactions are given in scheme 1.14.
 (1.14)
(1.14)

Fig. 1.2 The structures of solid state polymorphs of sulfur trioxide contains tetrahedral SO4 units: (a) γ-SO3 consists of trimeric units and (b) α- and β-SO3 contain polymeric chains. Colour code: S, yellow; O, red.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


