

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydrides H2E (E = S, Se, Te)
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 445
12-3-2017
1761
Hydrides H2E (E = S, Se, Te)
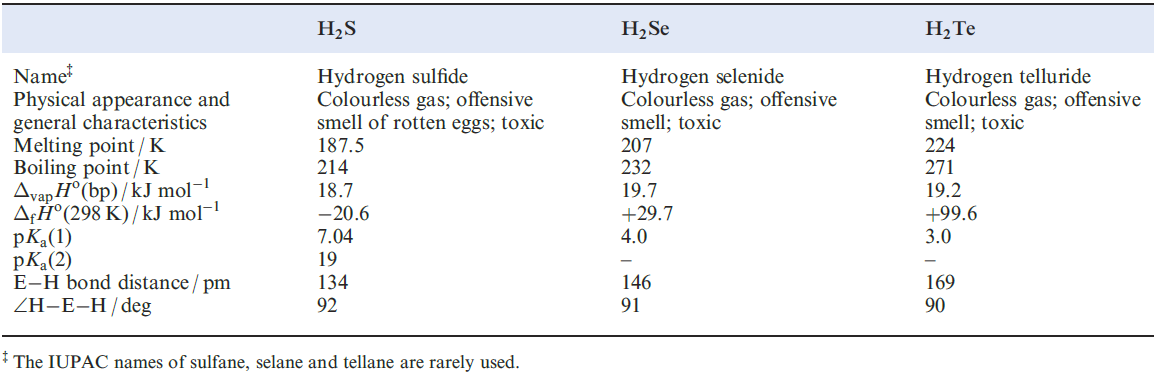
Selected physical data for hydrogen sulfide, selenide and telluride are listed in Table 1.1 and illustrated in Figures 9.6 and 9.7. Hydrogen sulfide is more toxic that HCN, but because H2S has a very characteristic odour of rotten eggs, its presence is easily detected. It is a natural product of decaying S-containing matter, and is present in coal pits, gas wells and sulfur springs. Where it occurs in natural gas deposits, H2S is removed by reversible absorption in a solution of an organic base and is converted to S by controlled oxidation. Figure 15.2 showed the increasing importance of sulfur recovery from natural gas as a source of commercial sulfur. In the laboratory, H2S was historically prepared by reaction 1.1 in a Kipp’s apparatus. The hydrolysis of calcium or barium sulfides (e.g. equation 1.2) produces purer H2S, but the gas is also commercially available in small cylinders.
 (1.1)
(1.1)
 (1.2)
(1.2)
Hydrogen selenide may be prepared by reaction 1.3, and a
similar reaction can be used to make H2Te.
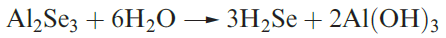 (1.3)
(1.3)
The enthalpies of formation of H2S, H2Se and H2Ten (Table 1.1) indicate that the sulfide can be prepared by direct combination of H2 and sulfur (boiling), and is more stable with respect to decomposition into its elements than H2Se or H2Te. Like H2O, the hydrides of the later elements in group 16 have bent structures but the angles of ≈ 90o (Table 1.1) are significantly less than that in H2O (1050). This suggests that the E_H bonds (E= S, Se or Te) involve p character from the central atom (i.e. little or no contribution from the valence s orbital). In aqueous solution, the hydrides behave as weak acids (Table 1.1 ).
Table 1.1 Selected data for H2S, H2Se and H2Te.
The second ionization constant of H2S is ≈ 10-19 and, thus, metal sulfides are hydrolysed in aqueous solution. The only reason that many metal sulfides
can be isolated by the action of H2S on solutions of their salts is that the sulfides are extremely insoluble. For example, a qualitative test for H2S is its reaction with aqueous lead acetate (equation 1.4).
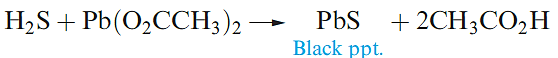 (1.3)
(1.3)
Sulfides such as CuS, PbS, HgS, CdS, Bi2S3, As2S3, Sb2S3 and SnS have solubility products less than ≈10-30 and can be precipitated by H2S in the presence of dilute HCl. The acid suppresses ionization of H2S, lowering the concentration of S2- in solution. Sulfides such as ZnS, MnS, NiS and CoS with solubility products in the range ≈ 10-15 to 10-30 are precipitated only from neutral or alkaline solutions. Protonation of H2S to [H3S]+ can be achieved using the superacid HF/SbF5. The salt [H3S][SbF6] is a white crystalline solid which reacts with quartz glass; vibrational spectroscopic data for [H3S]+ are consistent with a trigonal pyramidal structure like that of [H3O]+. The addition of MeSCl to [H3S][SbF6] at 77K followed by warming of the mixture to 213K yields [Me3S][SbF6], which is stable below 263 K. Spectroscopic data (NMR, IR and Raman) are consistent with the presence of the trigonal pyramidal [Me3S] cation.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















