

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Dichlorine, dibromine and diiodine
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 475
8-3-2017
1545
Dichlorine, dibromine and diiodine
Dichlorine is a pale green-yellow gas with a characteristic odour. Inhalation causes irritation of the respiratory system and liquid Cl2 burns the skin. Reaction 1.1 can be used for small-scale synthesis, but, like F2, Cl2 may be purchased in cylinders for laboratory use.
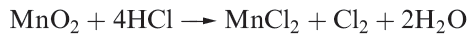 (1.1)
(1.1)
Dibromine is a dark orange, volatile liquid (the only liquid non-metal at 298 K) but is often used as the aqueous solution ‘bromine water’. Skin contact with liquid Br2 results in burns, and Br2 vapour has an unpleasant smell and causes eye and respiratory irritation. At 298 K, I2 forms dark purple crystals which sublime readily at 1 bar pressure into a purple vapour. In the crystalline state, Cl2, Br2 or I2 molecules are arranged in layers as represented in Figure 1.1. The molecules Cl2 and Br2 have intramolecular distances which are the same as in the vapour (compare these distances with rcov, Table 16.1). Intermolecular distances for Cl2 and Br2 are also listed in Figure 1.1; the distances within a layer are shorter than 2rv (Table 16.1), suggesting some degree of interaction between the X2 molecules. The shortest intermolecular X…..X distance between layers is significantly longer. In solid I2, the intramolecular I_I bond distance is longer than in a gaseous molecule, and the lowering of the bond order (i.e. decrease in intramolecular bonding) is offset by a degree of intermolecular bonding within each layer (Figure 1.1).

Fig. 1.1 Part of the solid state structures of Cl2, Br2 and I2 in which molecules are arranged in stacked layers, and relevant intramolecular and intermolecular distance data.
The molecules Cl2 and Br2 have intramolecular distances which are the same as in the vapour (compare these distances with rcov, Table 16.1). Intermolecular distances for Cl2 and Br2 are also listed in Figure 1.1; the distances within a layer are shorter than 2rv suggesting some degree of interaction between the X2 molecules. The shortest intermolecular X…..X distance between layers is significantly longer. In solid I2, the intramolecular I_I bond distance is longer than in a gaseous molecule, and the lowering of the bond order (i.e. decrease in intramolecular bonding) is offset by a degree of intermolecular bonding within each layer (Figure 1.1). It is significant that solid I2 possesses a metallic lustre and exhibits appreciable electrical conductivity at higher temperatures; under very high pressure I2 becomes a metallic conductor.
Chemical reactivity decreases steadily fromCl2 to I2, notably in reactions of the halogens with H2, P4, S8 and most metals. The values of Eo inTable 16.1 indicate the decrease in oxidizing power along the series Cl2 > Br2 > I2, and this trend is the basis of the methods of extraction of Br2 and I2 described in Section 16.2. Notable features of the chemistry of iodine which single it outamong the halogens are that it ismore easily:
- oxidized to high oxidation states;
- converted to stable salts containing I in the 1 oxidation state (e.g. Figure 16.4).
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















