

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Difluorine
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 474
8-3-2017
1414
Difluorine
Difluorine is a pale yellow gas with a characteristic smell similar to that of O3 or Cl2. It is extremely corrosive, being easily the most reactive element known. Difluorine is handled in Teflon or special steel vessels, although glass (see below) apparatus can be used if the gas is freed of HF by passage through sodium fluoride (equation 1.1).
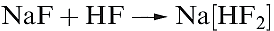 (1.1)
(1.1)
The synthesis of F2 cannot be carried out in aqueous media because F2 decomposes water, liberating ozonized oxygen (i.e. O2 containing O3); the oxidizing power of F2 is apparent from the Eo value listed in Table 16.1. The decomposition of a few high oxidation state metal fluorides generates F2, but the only efficient alternative to the electrolytic method used industrially is reaction 1.2. However, F2 is commercially available in cylinders, making laboratory synthesis generally unnecessary.
 (1.2)
(1.2)
Difluorine combines directly with all elements except O2, N2 and the lighter noble gases; reactions tend to be very violent. Combustion in compressed F2 ( fluorine bomb calorimetry) is a suitable method for determining values of ΔfHo for many binary metal fluorides. However, many metals are passivated by the formation of a layer of nonvolatile metal fluoride. Silica is thermodynamically unstable with respect to reaction 1.3, but, unless the SiO2 is powdered, the reaction is slow provided that HF is absent; the latter sets up the chain reaction 1.4.
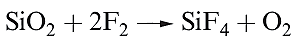 (1.3)
(1.3)
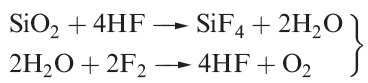 (1.4)
(1.4)
The high reactivity of F2 arises partly from the low bond dissociation energy (Figure 1.1) and partly from the strength of the bonds formed with other elements.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















