

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Silicon
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 518
4-3-2017
1495
Silicon
Silicon tetraalkyl and tetraaryl derivatives (R4Si), as well as alkyl or aryl silicon halides (RnSiCl4 - n, n = 1–3) can be prepared by reaction types 1.1–1.5. Note that variation in stoichiometry provides flexibility in synthesis, although the product specificity may be influenced by steric requirements of the organic substituents. Reaction 1.1 is used industrially (the Rochow process).
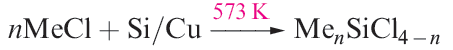 (1.1)
(1.1)
 (1.2)
(1.2)
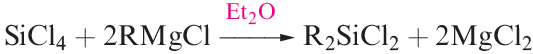 (1.3)
(1.3)
 (1.4)
(1.4)
The structures of these compounds are all similar: monomeric, with tetrahedrally sited Si and resembling their C analogues. Silicon_carbon bonds are relatively strong (the bond enthalpy term is 318 kJ mol-1) and R4Si derivatives possess high thermal stabilities. The stability of the Si_C bond is further illustrated by the fact that chlorination of Et4Si give ) ClCH2CH2)4Si, in contrast to the chlorination of R4Ge or R4Sn which yields RnGeCl4 - n or RnSnCl4- n (see equation 18.49). An important reaction of MenSiCl4_n (n = 1–3) is hydrolysis to produce silicones (e.g. equation 1.6).
 (1.5)
(1.5)
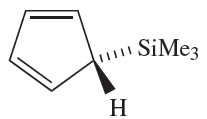
(1.1)
The reaction of Me3SiCl with NaCp leads to 1.1, in which the cyclopentadienyl group is η1. Related η1- complexes include) η1-C5Me5(2SiBr2 which reacts with anthracene/potassium to give the diamagnetic silylene(η5- C5Me5)2Si. In the solid state, two independent molecules are present (Figure 1.1a, b) which differ in the relative orientations of the cyclopentadienyl rings. In one molecule, the two C5-rings are parallel and staggered (compare Cp2Mg) whereas in the other, they are tilted (Figure 1.1c). We return to this observation at the end of Section 18.5. The reactions between R2SiCl2 and alkali metals or alkali metal naphthalides give cyclo-(R2Si)n by loss of Cl- and Si_Si bond formation. Bulky R groups favour small rings (e.g.(2,6-Me2C6H3)6Si3 and tBu6Si3) while smaller R substituents encourage the formation of large rings (e.g. Me12Si6;Me14Si7 and Me32Si16).
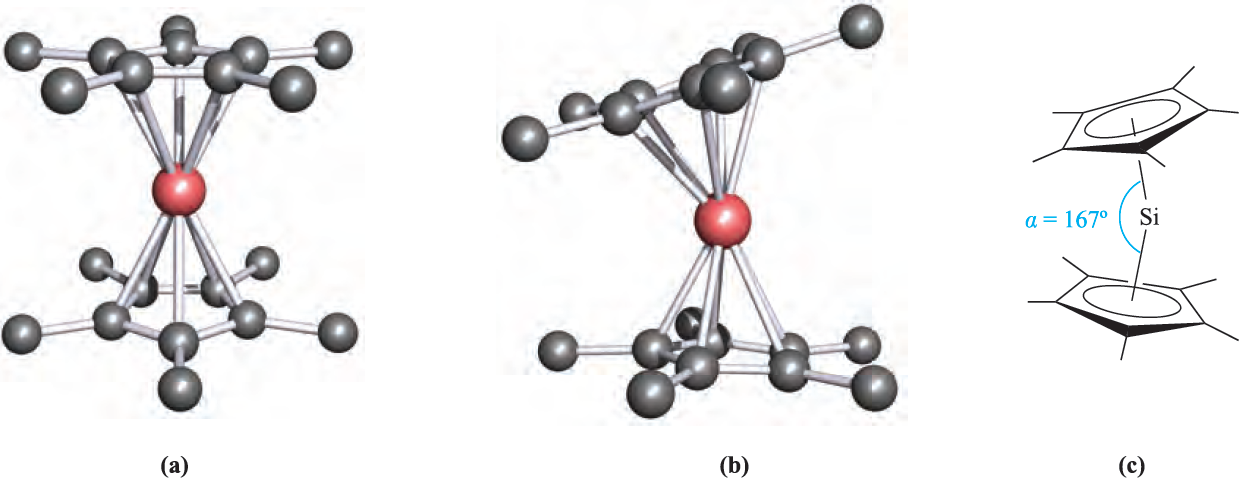
Fig. 1.1 The solid state structure of (η5-C5Me5(2Si contains two independent molecules. (a) In the first molecule, the cyclopentadienyl rings are co-parallel, while (b) in the other molecule they are mutually tilted; (c) the tilt angle is measured as angle α [P. Jutzi et al. (1986) Angew. Chem. Int. Ed. Engl., vol. 25, p. 164]. Hydrogen atoms are omitted for clarity; colour code: Si, pink; C, grey.
Reaction 1.6 is designed to provide a specific route to a particular ring size.
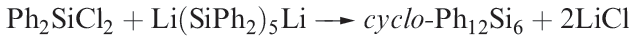 (1.6)
(1.6)
Silylenes, R2Si (analogues of carbenes), can be formed by a variety of methods, for example, the photolysis of cyclic or linear organopolysilanes. As expected, R2Si species are highly reactive, undergoing many reactions analogous to those typical of carbenes. Stabilization of R2Si can be achieved by using sufficiently bulky substituents, and electron diffraction data confirm the bent structure of {(Me3Si)2HC}2Si (∠C_Si_C = 970). The sterically demanding 2,4,6-iPr3C6H2 group has been used to stabilize 1.2, the first example of a compound containing conjugated Si=Si bonds. An unusual feature of 1.2 is the preference for the s-cis conformation in both solution and the solid state.
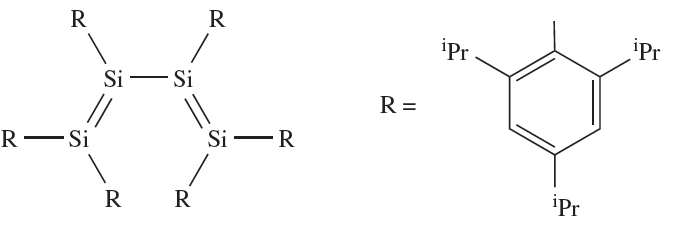
(1.2)
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















