


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 1-10-2020
Date: 4-10-2020
Date: 20-12-2020
|
Coulomb’s Law
In 1785, Coulomb established the fundamental law of electric force between two stationary, charged particles. Experiments show that an electric force has the following properties:
(1) The force is inversely proportional to the square of separation, r2, between the two charged particles.
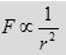
(2) The force is proportional to the product of charge q1 and the charge q2 on the particles.
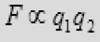
(3) The force is attractive if the charges are of opposite sign and repulsive if the charges have the same sign.
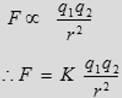
where K is the coulomb constant = 9 × 109 N.m2/C2.
The above equation is called Coulomb’s law, which is used to calculate the force between electric charges. In that equation F is measured in Newton (N), q is measured in unit of coulomb (C) and r in meter (m).
The constant K can be written as
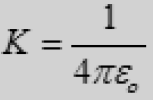
where εο is known as the Permittivity constant of free space.
□ εο = 8.85 × 10-12 C2/N.m2
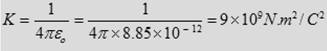



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|