


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-1-2017
Date: 25-2-2018
Date: 19-4-2017
|
Percent yield
In almost any reaction, you’re going to produce less of the product than you expected. You may produce less because most reactions are equilibrium reactions, because of sloppy technique or impure reactants, or because some other conditions come into play. Chemists can get an idea of the efficiency of a reaction by calculating the percent yield for the reaction using this equation:

The actual yield is how much of the product you get when you carry out the reaction. The theoretical yield is how much of the product you calculate you’ll get. The ratio of these two yields gives you an idea about how efficient the reaction is.
For the reaction of rust to iron (see the preceding section), your theoretical yield is 699.5 grams of iron; suppose your actual yield is 525.0 grams. Therefore, the percent yield is
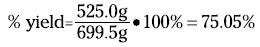
A percent yield of about 75 percent isn’t too bad, but chemists and chemical engineers would rather see 90+ percent. One industrial plant using the Haber reaction has a percent yield of better than 99 percent. Now that’s efficiency!



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|