
The dreaded energy level diagram
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p 29
الجزء والصفحة:
p 29
 28-12-2016
28-12-2016
 2712
2712
The dreaded energy level diagram
Figure 1.1 is a blank energy level diagram you can use to depict electrons for any particular atom. It doesn’t show all the known orbitals and subshells, but with this diagram, you should be able to do most anything you need to.
I represent orbitals with dashes in which you can place a maximum of two electrons. The 1s orbital is closest to the nucleus, and it has the lowest energy. It’s also the only orbital in energy level 1. At energy level 2, there are both s and p orbitals, with the 2s having lower energy than the 2p. The three 2p subshells are represented by three dashes of the same energy. The figure also shows energy
levels 3, 4, and 5.
Notice that the 4s orbital has lower energy than the 3d: This is an exception to what you may have thought, but it’s what’s observed in nature. Go figure.
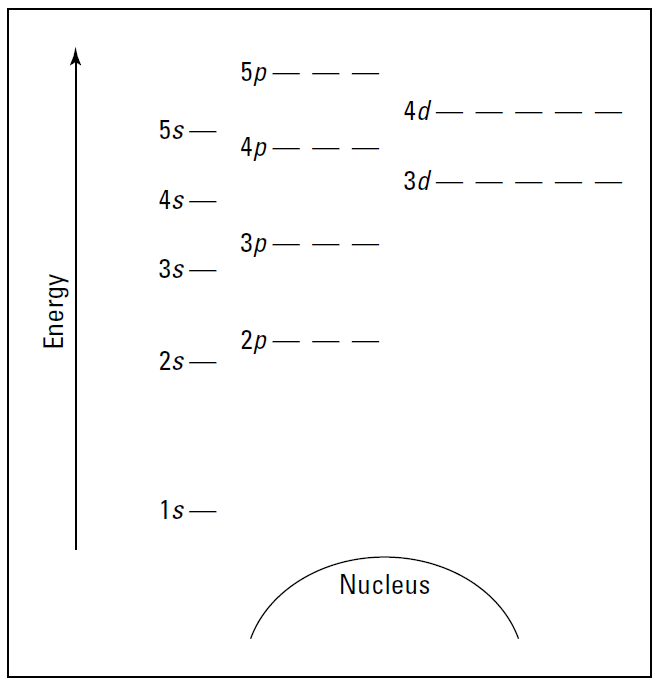
Figure 1.1: An energy level diagram.
Speaking of which, Figure 1.2 shows the Aufbau principle, a method for remembering the order in which orbitals fill the vacant energy levels.
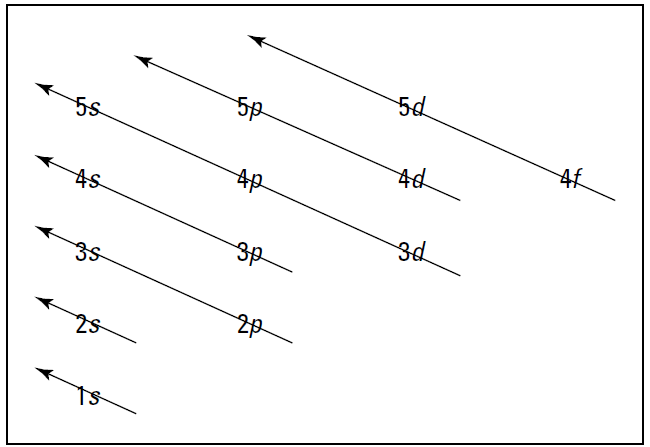
Figure 1.2: The Aufbau filling chart.
In using the energy level diagram, remember two things:
✓ Electrons fill the lowest vacant energy levels first.
✓ When there’s more than one subshell at a particular energy level, such as at the 3p or 4d levels (see Figure 1.1), only one electron fills each subshell until each subshell has one electron. Then electrons start pairing up in each subshell. This rule is named Hund’s rule.
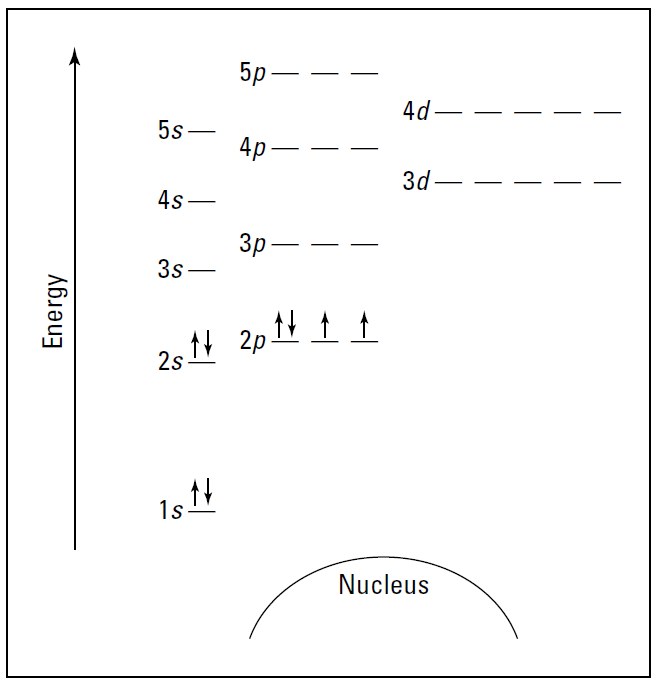
Suppose you want to draw the energy level diagram of oxygen. You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has eight protons in its nucleus and eight electrons. So you put eight electrons into your energy level diagram. You can represent electrons as arrows, as in Figure 1.3. Note that if two electrons end up in the same orbital, one arrow faces up and the other faces down.
This is called spin pairing. It corresponds to the +1⁄2 and –1⁄2 of ms (see “The spin quantum number ms” section, earlier in this chapter, for details). The first electron goes into the 1s orbital, filling the lowest energy level first, and the second one spin-pairs with the first one. Electrons 3 and 4 spin-pair in the next-lowest vacant orbital — the 2s. Electron 5 goes into one of the 2p subshells (no, it doesn’t matter which one — they all have the same energy), and electrons 6 and 7 go into the other two totally vacant 2p orbitals. The last electron spin-pairs with one of the electrons in the 2p subshells (again, it doesn’t matter which one you pair it with). Figure 1.3 shows the completed energy level diagram for oxygen.
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


