


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-4-2019
Date: 17-2-2016
Date: 19-2-2019
|
The quantum mechanical model
Early models of the atom had electrons going around the nucleus in a random fashion. But as scientists discovered more about the atom, they found that this representation probably wasn’t accurate. Today, scientists use the quantum mechanical model, a highly mathematical model, to represent the structure of the atom.
This model is based on quantum theory, which says that matter also has properties associated with waves. According to quantum theory, it’s impossible to know an electron’s exact position and momentum (speed and direction, multiplied by mass) at the same time. This is known as the uncertainty principle. So scientists had to develop the concept of orbitals (sometimes called electron clouds), volumes of space in which an electron is likely present. In other words, certainty was replaced with probability.
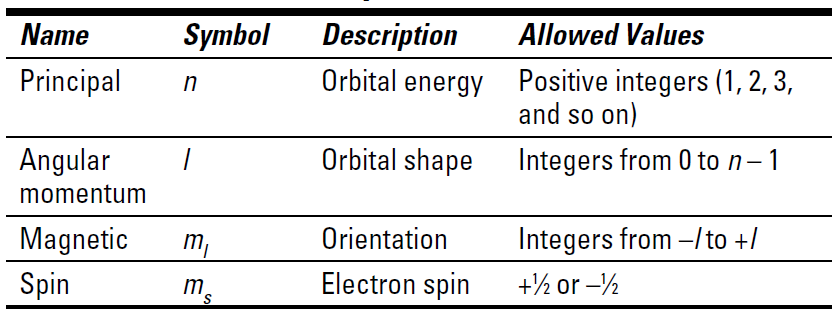
The quantum mechanical model of the atom uses complex shapes of orbitals. Without resorting to a lot of math (you’re welcome), this section shows you some aspects of this newest model of the atom. Scientists introduced four numbers, called quantum numbers, to describe the characteristics of electrons and their orbitals. You’ll notice that they were named by top-rate techno-geeks:
✓ Principal quantum number n
✓ Angular momentum quantum number l
✓ Magnetic quantum number ml
✓ Spin quantum number ms
Table 1-1 summarizes the four quantum numbers. When they’re all put together, theoretical chemists have a pretty good description of the characteristics of a particular electron.
Table 1-1 Summary of the Quantum Numbers



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|