

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
aziridines
المؤلف:
B.U.W. Maes · J. Cossy · S. Polanc
المصدر:
Structure, Bonding and Reactivity of Heterocyclic Compounds
الجزء والصفحة:
p 3
15-12-2016
2167
aziridines
The aziridine moiety A (Fig. 1) represents one of the most valuable threemembered ring systems in organic chemistry, due to the uncommon combination of reactivity, synthetic flexibility, and atom economy. Indeed, ring strain renders aziridines susceptible to ring-opening reactions that dominate their chemistry and makes them useful synthetic intermediates in the arsenal of the organic chemist. The regio-controlled ring opening of C-substituted aziridines constitutes a powerful approach toward the preparation of a large variety of functionalized nitrogen-containing target compounds. The ring opening of activated aziridines, i.e., aziridines with an electron-withdrawing group at the nitrogen atom, has shown to be quite straightforward, mostly involving the nucleophilic attack at the less-hindered aziridine carbon atom.
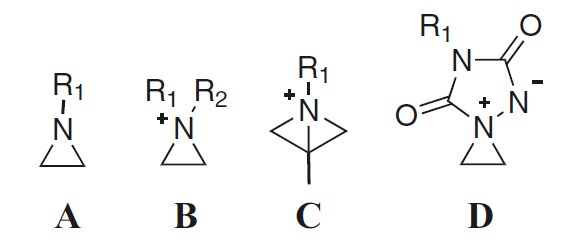
Fig. 1 Aziridines A, aziridinium ions B and C, and aziridinium ylides D
On the other hand, nonactivated aziridines, which do require quaternization toward an aziridinium intermediate B for nucleophilic ring-opening reactions due to an electron-donating group at the nitrogen atom, often have different reactivity and applications. They provide interesting opportunities for the selective synthesis of a variety of functionalized amines. It should be noted that aziridinium ions not only can be obtained through N-functionalization of neutral aziridines but also through intramolecular substitution of amines bearing a leaving group at the β-position.
Their are different types of strain and different methods to determine ring strain are described and a rationalization of the ring strain of different aziridinium ions will be given based on theoretical calculations. Next, a short overview will be given of the formation of aziridinium ions B from both the rearrangement of β-amino alcohols and the ring opening of nonactivated aziridines, and the reactivity of aziridinium ions will be investigated theoretically. In addition, the role of bicyclic aziridinium ions C as intermediates in the ring expansion of aziridines to azetidines will be unraveled. In the last part, we highlight the importance of spiro aziridinium ylides D as intermediates in ene reactions involving the highly reactive electrophile triazolinedione.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















