
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Laser action caused by molecular oscillations
المؤلف:
H. HAKEN
المصدر:
LASER LIGHT DYNAMICS
الجزء والصفحة:
vol 2 ، p33
7-12-2016
2578
Laser action caused by molecular oscillations
The most important example is provided by the CO2 gas laser. In these molecules the individual atoms can perform oscillations. The three fundamental kinds of oscillations are shown in fig. 1.1. According to quantum theory the different kinds of oscillations must be quantized so that discrete energy levels result. The energy level diagram belonging to some low lying oscillation levels of CO2 is represented in fig. 1.2. One of the laser processes rests on the optical transition between the levels which are denoted in fig. 1.2 by 00'1 and 10'0. The excitation of the uppermost level is usually achieved in a plasma discharge in which N2 and He participate in addition to CO2. In the plasma discharge a large fraction of the two-atomic N2 molecules is excited to make vibrations, whereby the molecules accumulate in the excited state with the vibration quantum number n = 1 of the harmonic oscillator. Collisions with CO2 molecules in their ground states make a transfer of the energy from the excited state of N2 to an excited state of CO2 possible. The remaining small energy difference is transformed into kinetic energy of the molecules after their collisions. The efficiency of CO2 lasers is very high and lies at about 30%. In order to achieve high power emission, lasers with a length up to several hundred meters have been built. According to quantum theory, besides the vibrational levels of CO2 molecules also discrete rotational levels are possible which may also participate in the laser process. If the gas pressure is increased above 5 Torr, due to the numerous collisions a line-broadening occurs which exceeds the usual


Fig. 1.1. Oscillatory states of the CO2 molecule.
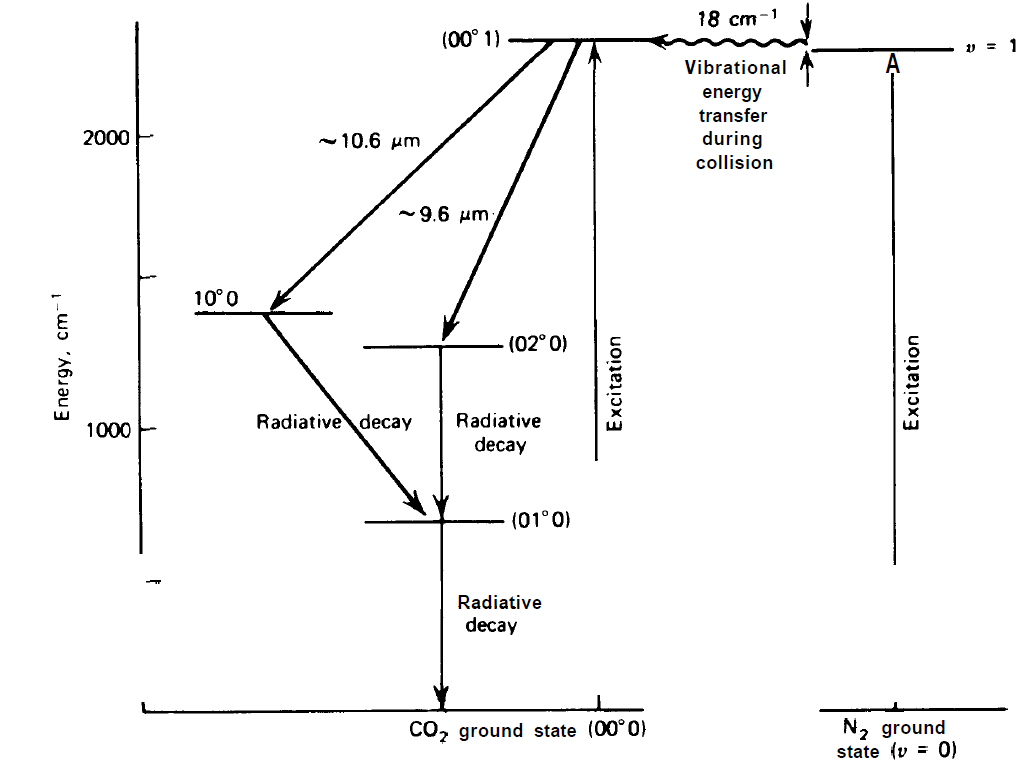
Fig. 1.2. Oscillatory states of CO,.


Fig. 1.3. Typical arrangement of a gas dynamic laser.
Doppler broadening. This gives rise to a second laser regime of the CO2 laser which is of particular interest for applications. Particularly high emission powers can be achieved by gas dynamic lasers. Here a mixture of CO2, N,, H2O or He is used. This gas mixture, which is initially held under high pressure and is very hot, can expand through supersonic jets. During expansion an inversion of the gas atoms is reached so that a laser active medium results. The supersonic gas passes through an arrangement of mirrors whereby a laser light beam is generated (cf. fig. 1.3).
 الاكثر قراءة في مواضيع عامة في الليزر
الاكثر قراءة في مواضيع عامة في الليزر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















