


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 18-8-2016
Date: 21-8-2016
Date: 21-8-2016
|
Joule Cycle
Find the efficiency of the Joule cycle, consisting of two adiabats and two isobars (see Figure 1.1). Assume that the heat capacities of the gas CP and CV are constant.
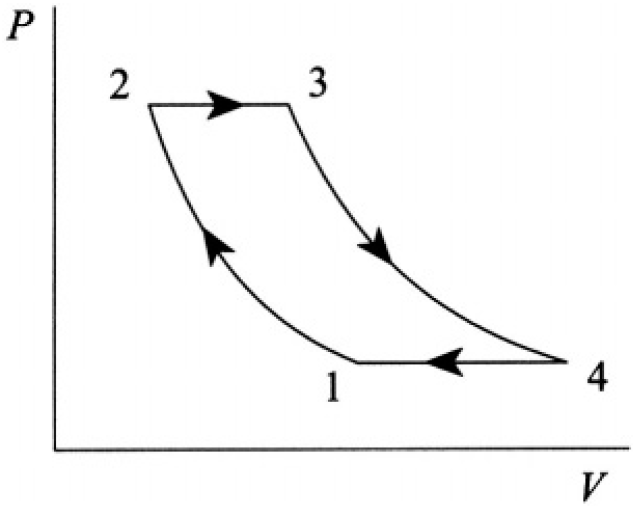
Figure 1.1
SOLUTION
The efficiency η of the cycle is given by the work W during the cycle divided by the heat Q absorbed in path 2 → 3 (see Figure 1.2). W is defined by the area enclosed by the four paths of the P–V plot. The integral ∫P dV along the paths of constant pressure 2 → 3 and 4→1 is simply the difference in volume of the ends times the pressure, and the work along the adiabats, where there is no heat transfer δQ = 0, is given by the change in internal energy CVδT:
 (1)
(1)

Figure 1.2
Substituting the ideal gas law PV = nRT into (1) and rearranging, we find
 (2)
(2)
where we used CP = CV + nR. In the process 2 → 3, the gas absorbs the heat Q:
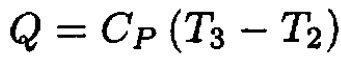 (3)
(3)
What remains is to write W and Q in terms of P and form the quotient. Using the equation for an adiabatic process in an alternative form,
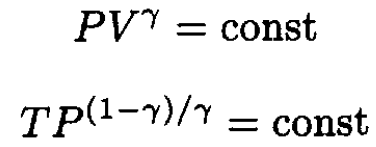
we have
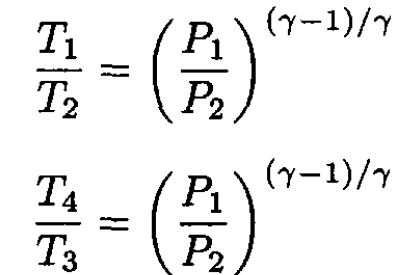 (4)
(4)
Substituting for T1 and T4 by putting (4) into (1) yields
 (5)
(5)
The efficiency η is then




|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|