


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 19-8-2016
Date: 22-8-2016
Date: 11-8-2016
|
Air Bubble Coalescence
A tightly closed jar is completely filled with water. On the bottom of the jar are two small air bubbles (see Figure 1.1a) which sidle up to each other and become one bubble (see Figure 1.1b). The pressure at the top of the jar is P0, the radius of each original bubble is R0, and the coefficient of surface tension is σ. Consider the process to be isothermal. Evaluate the change of pressure inside the jar upon merging of the two bubbles.

Figure 1.1
SOLUTION
Writing the equilibrium conditions for the bubbles to exist, we find for the pressure inside each original bubble:
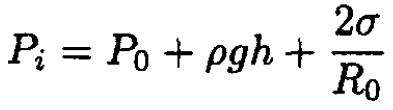 (1)
(1)
where ρgh is the hydrostatic pressure (h is the height of the water). We disregard any effects due to the finite size of the bubble since they are small (R0 << h). After merging, the pressure inside the new bubble will not change. This is due to the fact that the temperature is constant, and since the jar is closed and water is incompressible, the total volume also will not change. The new radius R1 is given by
 (2)
(2)
Writing (S.4.61.1) for the new bubble, we obtain
 (3)
(3)
where we disregard any small change in hydrostatic pressure. From (1) and (3) we find that the change of pressure inside the jar is
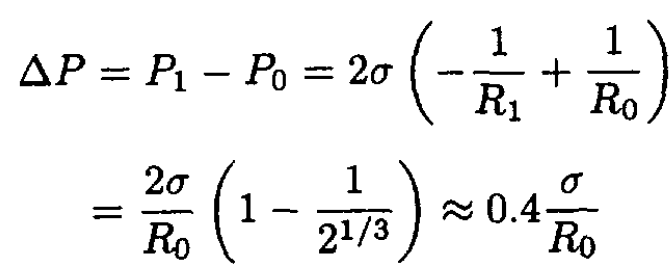 (4)
(4)



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|